Stem Cell Therapy (ESC, MSC, IPSC, HSC in vivo transplantation)
Cell therapy is a kind of medicine aiming to cure disease or alleviate disease symptoms via direct infusion or transplantation of cells, which can be autologous or allogeneic. With several decades’ development and optimization, immuno-oncology cells (such as T cells, nature killer cells, etc.), stem cells (embryonic stem cells, induced pluripotent stem cells, progenitor cells, etc.) or other genetic re-engineered cells have been widely applied for cell therapy. Numerous cell types have been translated into clinical trials and promising cell therapy outcomes have been achieved from Phase I, Phase II and Phase III trials for a great number of diseases.
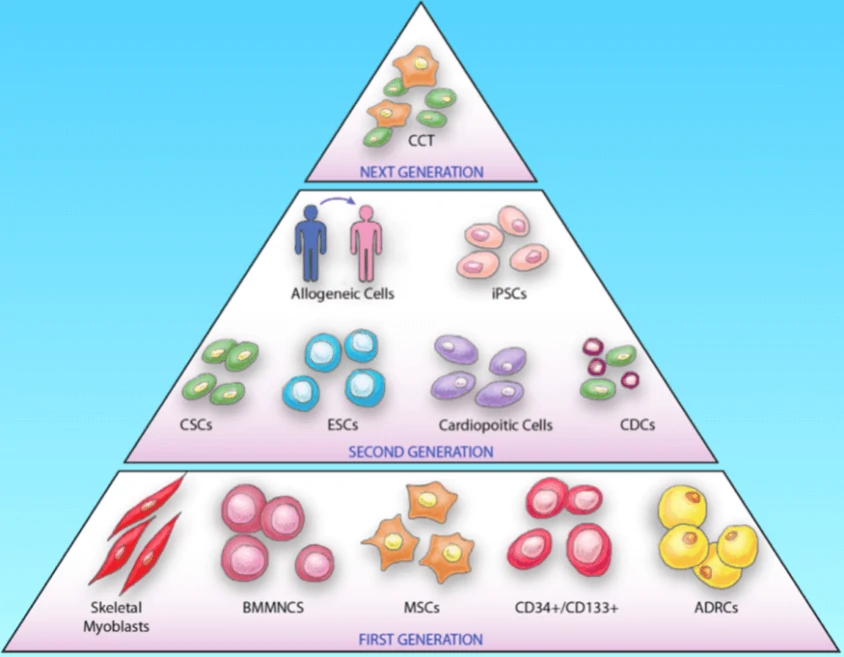
Cell therapy based on stem cells is a kind of regenerative medicine with great potential for the treatment of diseases via the differentiation ability or the paracrine effects of stem cells as well as their derivatives. To date, a great number of cell types, have been applied from bench to bedside to treat diseases, such as cardiovascular diseases, neurodegenerative diseases and cancers. The cell types can be classified into three generations (Fig. 8): the 1st generation consists of skeletal myoblasts, bone marrow mononuclear cells (BMMNCs) and adipose-derived regenerative cells (ADRCs), mesenchymal stem cells (MSCs) and hematopoietic stem cells (CD34+/CD133+); the 2nd generation mainly utilizes embryonic stem cells (ESCs), induced pluripotent stem cells (iPS), cardiac stem cells (CSC) etc.; the next generation focuses on the combination therapy (CCT) of specific cell types, such as cell mixtures of cardiomyocytes, endothelial cells and smooth muscle cells.
MSC
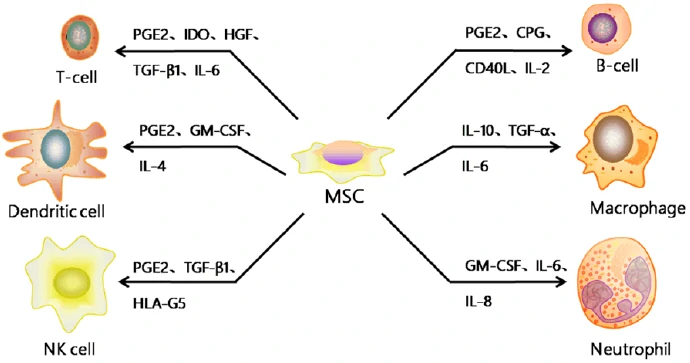
Mesenchymal stromal/stem cells (MSC) are stromal cells in almost all the tissues or organs, identified by the expression of a specific panel of cell surface markers (CD1051, CD731, CD901, CD34-, CD14-, or CD11b-, CD79- or CD19-, HLA-DR-) and the differentiation potentials into at least osteogenic, adipogenic, and chondrogenic lineages [92]. Although MSCs exist in almost all the human tissues, most of the MSCs used for clinical trials are isolated from bone marrow, adipose tissue and umbilical cord blood. And MSCs may have different biological characteristics and differentiation tendency according to their tissue sources as well as the isolation and culture procedures [93,94]. Based on the strong immunomodulatory, anti-inflammatory, and proregenerative capacities (Fig. 9) [95], MSCs have been promising candidates for the treatment of neurodegenerative diseases, Graft-versus-host disease (GvHD) and so on (Table 5) [96].
| Disease | Delivery route | Clinical trials | Outcome | Reference |
| Amyotrophic lateral sclerosis (ALS) | Intrathecally | Phase I | No adverse effects | [97] |
| Amyotrophic lateral sclerosis (ALS) | Intramuscularly, intrathecally | Phase I/II | No adverse effects | [98] |
| Amyotrophic lateral sclerosis (ALS) | Intrathecally | Phase I | No adverse effects | [99] |
| Amyotrophic lateral sclerosis (ALS) | Intrathecally | Phase I/IIa | No adverse effects | [100] |
| Multiple sclerosis (MS) | Intravenously | Phase I/II | Intial safety profile and feasibility of the intervention | [101] |
| Multiple sclerosis (MS) | Intravenously | Phase I/IIa | Improvement of visual acuity | [102] |
| Multiple sclerosis (MS) | Intravenously | Phase I/IIa | Reduction in inflammation | [103] |
| ALS/MS | Intrathecally, intravenously | Phase I/II | Induces rapid immunomodulatory effects | [104] |
| Spinal cord injury | Intravenously | Phase I | No tumor development | [105] |
| Spinal cord injury | Locally injection | Phase III | Variable improvements in tactile sensitivity | [106] |
| Spinal cord injury | intramedullary, subdurally | Phase III | Weak therapeutic effect | [107] |
| Osteoarthritis | Intraarticulary | Phase I | Improvement of function and pain in high dose group | [108] |
| Osteoarthritis | Intraarticulary | Phase I | Improvement in pain levels in low dose group | [109] |
| Graft-versus-host disease (GvHD) | Intravenously | Phase I/II | No adverse effects | [110] |
| Graft-versus-host disease (GvHD) | Intravenously | Phase I | Immunosuppressive therapy damaged intestinal epithelium | [111] |
| Graft-versus-host disease (GvHD) | Intravenously | Phase I/II | Clinical responses | [112] |
| Crohn’s disease | Intravenously | Phase I | No adverse effects | [113] |
| Crohn’s disease | Intravenously | Phase II | Reduce Crohn’s disease activity index | [114] |
| Liver failure | Intravenously | Phase I/II | Reduce Model for End-stage Liver Disease (MELD) score | [115] |
| Liver cirrhosis | Intravenously | Phase I | Improvement in MELD score | [116] |
| Kidney disease | Intravenously | Phase I | Systemic immunosuppression | [117] |
| Acute myocardial infarction | Infarcted sites | Phase I/II | No adverse effects | [118] |
| Acute myocardial infarction | Infarct-related artery | Phase II/III | Modest improvement in left ventricular ejection fraction (LVEF) at 6-month follow-up | [119] |
ESCs/iPSCs or their derived cells
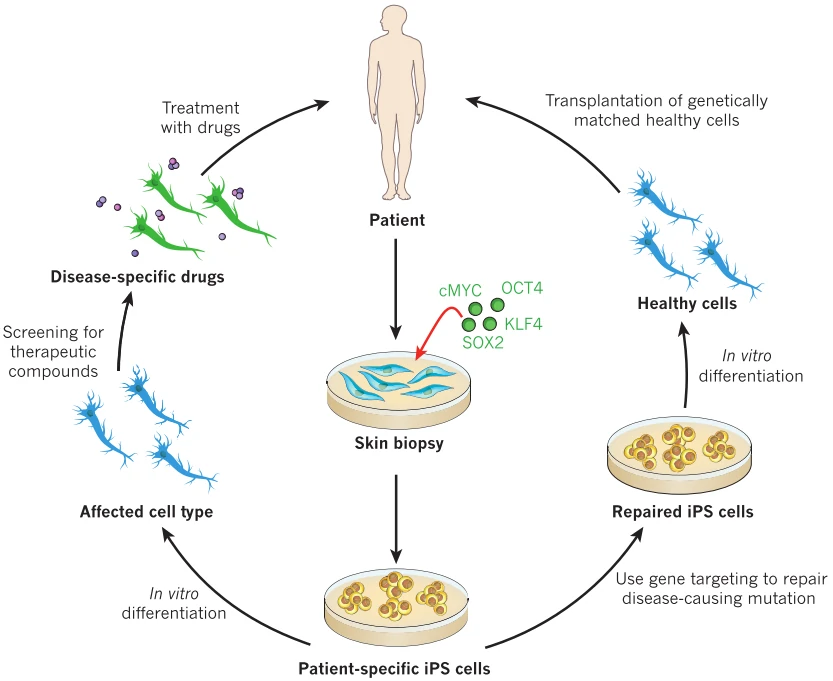
With the ability to self-renewal and differentiation into three germ layer derived lineages, embryonic stem cells (ESCs) [120-122] and induced pluripotent stem cells (iPSCs) [123,124] were thought to be invaluable candidates for disease regenerative therapy, especially iPSCs with no or mild immunological rejection (Fig. 10). However, their tumorigenicity greatly limits the direct transplantation of ESCs/iPSCs to treat disease [125]. Then lineage directed progenitor cells, such as neural progenitor/stem cells [126] and cardiac progenitor/stem cells [127], or terminally differentiated cells, such as cardiomyocytes [128,129], endothelial cells [130], smooth muscle cells [131], and retinal pigment epithelium[132] have been widely used in the therapy of a variety of diseases (Table 6).
| Disease | Cell types | Phase of clinical trials | Delivery route | Outcome | Reference |
| Ischemic Cardiomyopathy | CSCs | Phase I | intracoronary | LVEF increased, scar size reduced, life quality improved | [134] |
| Ischemic Cardiomyopathy | cardiosphere-derived cells (CDCs) | Phase I | intracoronary | Scar size reduced | [135] |
| Ischemic Cardiomyopathy | Cardiopoietic cells | Phase II | transendocardial stem cell injection | No significance | [136] |
| Nonischemic Cardiomyopathy | CD34+ stem cell | Phase II | intracoronary | LVEF increased, function capacity improved | [137], [138] |
| Macular degeneration | hESC-derived retinal pigment epithelium | Phase I/II | subretinal injection | increase in subretinal pigmentation, vision-related life quality improved | [132], NCT01344993 |
| Stargardt’s macular dystrophy | hESC-derived retinal pigment epithelium | Phase I/II | subretinal injection | increase in subretinal pigmentation, vision-related life quality improved | [132], NCT01345006 |
| Amyotrophic lateral sclerosis (ALS) | neural stem cells | Phase I | intraspinal | well tolerated | [139] |
| Amyotrophic lateral sclerosis (ALS) | neural stem cells | Phase I | intraspinal | well-tolerated | [140] |
| Amyotrophic lateral sclerosis (ALS) | neural stem cells | Phase I | intraspinal | safe | [141] |
| Parkinson’s disease | human parthenogenetic stem cell-derived NSCs (hpNSCs) | Phase I | striatum | dopamine levels increase | [142] |
| Stroke | neural stem cells | Phase I | stereotactic ipsilateral putamen injection | neurological function improved | [143] |
Other Cell Therapy
Cell Combination Therapy (CCT) is also a promising therapy with the combination of several cell types, resulting in better therapy outcomes covering the advantages of different cell types. The combination of autologous CSCs and MSCs displayed better cardioreparative effects on swine ischemic cardiomyopathy model than that of MSCs [144,145]. Tri-lineage cell transplantation (cardiomyocytes, endothelial cells, and smooth muscle cells) displayed significant cardiac repair effects in the porcine acute myocardial infarction model by improving left ventricular function, myocardial metabolism, and arteriole density, as well as reducing infarct size and cardiomyocyte apoptosis [146]. Moreover, numerous biocompatible scaffolds, such as hydrogels [147], were used to facilitate the grafts integration in the compromised disease microenvironment and improve the survival of transplanted cells [148], which might significantly promote the clinical application of cell therapy.




