1. Ryu, S., et al., One Health Perspectives on Emerging Public Health Threats. J Prev Med Public Health, 2017. 50(6): p. 411-414.
2. World Health Organization (WHO). Zoonoses. Accessed October 3, 2018.; Available from: http://www.who.int/zoonoses.
3. Leslie MJ, M.J. Surveillance for zoonotic diseases. BLUKO97- Mikanatha 2007; Available from: http://courses.washington.edu/zepi526/Papers08/Rabies%20chapter.pdf.
4. Available from: https://www.doctorsgate.com/en/what-are-the-current-diagnostic-tests-for-covid-19/.
5. Anylab. Available from: www.zetbio.com.
6. Li, H., et al., A new and rapid approach for detecting COVID-19 based on S1 protein fragments. Clin Transl Med, 2020. 10(2): p. e90.
7. Khanna, M., et al., Evaluation of influenza virus detection by direct enzyme immunoassay (EIA) and conventional methods in asthmatic patients. J Commun Dis, 2001. 33(3): p. 163-9.
8. Waner, J.L., et al., Comparison of Directigen FLU-A with viral isolation and direct immunofluorescence for the rapid detection and identification of influenza A virus. J Clin Microbiol, 1991. 29(3): p. 479-82.
9. Cameron, J.D., A.P. Skubitz, and L.T. Furcht, Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest Ophthalmol Vis Sci, 1991. 32(10): p. 2766-73.
10. Kim, M.H., S.Y. Kang, and W.I. Lee, Evaluation of a new rapid test kit to detect hepatitis C virus infection. J Virol Methods, 2013. 193(2): p. 379-82.
11. Niu, X., et al., [Establishment of the evaluation reference system for domestic anti-hepatitis C virus diagnostic enzyme immunoassay kits]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 2013. 29(7): p. 761-4.
12. Filice, G., et al., Sensitivity and specificity of anti-HIV ELISA employing recombinant (p24, p66, gp120) and synthetic (gp41) viral antigenic peptides. Microbiologica, 1991. 14(3): p. 185-94.
13. de Boer, G.F., W. Back, and A.D. Osterhaus, An ELISA for detection of antibodies against influenza A nucleoprotein in humans and various animal species. Arch Virol, 1990. 115(1-2): p. 47-61.
14. Cuzzubbo, A.J., et al., Comparison of PanBio dengue duo enzyme-linked immunosorbent assay (ELISA) and MRL dengue fever virus immunoglobulin M capture ELISA for diagnosis of dengue virus infections in Southeast Asia. Clin Diagn Lab Immunol, 1999. 6(5): p. 705-12.
15. Maria-C-Jimenez-Martinez. Available from:
https://www.researchgate.net/profile/Maria-C-Jimenez-Martinez/publication/320265684/figure/fig3/AS:547016444395520@1507430291554/ELISA-assays-Direct-ELISA-mostly-used-for-antigen-detection-Indirect-ELISA-mainly-used.webp.
16. sciencephoto. Available from: https://www.sciencephoto.com/media/90184/view.
17. Uyeki, T.M., et al., Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin Infect Dis, 2019. 68(6): p. e1-e47.
18. Goldsmith, C. Hantavirus Life Cycle and Infection Process. Available from: https://www.hantasite.com/2017/03/hantavirus-life-cycle-and-infection.html.
19. METHODS, M. 2013; 3:207
20. biotech, G.; Available from: https://www.globalbiotechinsights.com/articles/20247/the-worldwide-test-for-covid-19.
Veterinary diagnostics antibodies and antigens for animal health test kit, animal infectious diseases diagnostics and veterinary/Zoonotic diagnostic laboratory in ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA),turbidimetric inhibition immuno assay (TINIA) and POCT
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

Full product list: Ruminants, Companion animal, Swine, Equine, Avian, Fish, Multiple Species
Livestock, poultry, and aquaculture are among the fastest growing and expanding agriculture sectors to fulfill the need of the growing population of humans. However, the growth in this sector is under the continuous increasing threats of infectious diseases worldwide. This menace is further aggravated by globalization in animal trade for various purposes. The sudden entry of an infectious disease in a new country or geographical location could lead to delayed diagnosis and rapid spread into the susceptible animal population. Hence, Animal diagnostics are critical for animal health, identifying health issues before they are otherwise able to be detected and supporting faster diagnosis and treatment planning. Diagnosis is an essential part of disease management and prevention. The importance of animal disease diagnostics laboratories is not a very recognized area of animal production but they are essential to animal health. It is important for not only animal producers, but also consumers to be aware of this resource. The application of innovative diagnostic technologies for the detection of animal pathogens at an early stage is essential in restricting the economic loss incurred due to emerging infectious animal diseases. The desirable characteristics of such diagnostic methods are easy to use, cost-effective, highly sensitive, and specific, coupled with the high-throughput detection capabilities. Genemedi provides diagnostic antibodies and antigens for the in vitro diagnosis of diseases from the companion animal, Cat/Feline, Dog/Canine, Rabbit, Bovines/Cattle, Ovines/Sheep, Caprine/Goat, Equine/Horse, Swine/Porcine/Pig, Avian/bird/poultry, Fish and so on.
Cat No. | Specific Host | Pathogen | Target | Disease | Cat No.of Antigen | Bioactivity validation of Antigen | Cat No.of Antibodies | Bioactivity validation of Antibodies | Order |
|---|---|---|---|---|---|---|---|---|---|
Cat/Feline, Dog/Canine |
Ehrlichia canis |
P30 |
Ehrlichiosis |
GMP-VT-P029-Tg001-Ag001 |
Anti-Ehrlichia canis P30 antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Ehrlichia canis level test of animal Cat/Feline, Dog/Canine infectious disease with Ehrlichiosis. |
GMP-VT-P029-Tg001-Ab001/
GMP-VT-P029-Tg001-Ab002 |
Recombinant Ehrlichia canis P30 antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Ehrlichia canis antibodies in Ehrlichia canis level test of animal Cat/Feline, Dog/Canine infectious disease with Ehrlichiosis. |
||
Dog/Canine |
Canine parvovirus/CPV/CPV2,/parvo |
VP2 |
Canine parvovirus infection |
GMP-VT-P030-Tg001-Ag001 |
Anti-Canine parvovirus/CPV/CPV2,/parvo VP2 antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Canine parvovirus/CPV/CPV2,/parvo level test of animal Dog/Canine infectious disease with Canine parvovirus infection. |
GMP-VT-P030-Tg001-Ab001/
GMP-VT-P030-Tg001-Ab002 |
Recombinant Canine parvovirus/CPV/CPV2,/parvo VP2 antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Canine parvovirus/CPV/CPV2,/parvo antibodies in Canine parvovirus/CPV/CPV2,/parvo level test of animal Dog/Canine infectious disease with Canine parvovirus infection. |
||
Dog/Canine |
Anaplasma platys |
OMP-1X |
canine cyclic thrombocytopenia, or thrombocytotropic anaplasmosis |
GMP-VT-P031-Tg001-Ag001 |
Anti-Anaplasma platys OMP-1X antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Anaplasma platys level test of animal Dog/Canine infectious disease with canine cyclic thrombocytopenia, or thrombocytotropic anaplasmosis. |
GMP-VT-P031-Tg001-Ab001/
GMP-VT-P031-Tg001-Ab002 |
Recombinant Anaplasma platys OMP-1X antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Anaplasma platys antibodies in Anaplasma platys level test of animal Dog/Canine infectious disease with canine cyclic thrombocytopenia, or thrombocytotropic anaplasmosis. |
||
Dog/Canine, Equine/Horse |
Anaplasma platys |
0 |
thrombocytopenia |
GMP-VT-P031-Tg002-Ag001 |
Anti-Anaplasma platys antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Anaplasma platys level test of animal Dog/Canine, Equine/Horse infectious disease with thrombocytopenia. |
GMP-VT-P031-Tg002-Ab001/
GMP-VT-P031-Tg002-Ab002 |
Recombinant Anaplasma platys antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Anaplasma platys antibodies in Anaplasma platys level test of animal Dog/Canine, Equine/Horse infectious disease with thrombocytopenia. |
||
Cat/Feline |
Bartonella henselae |
P26 |
Cat scratch disease (CSD) |
GMP-VT-P032-Tg001-Ag001 |
Anti-Bartonella henselae P26 antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Bartonella henselae level test of animal Cat/Feline infectious disease with Cat scratch disease (CSD). |
GMP-VT-P032-Tg001-Ab001/
GMP-VT-P032-Tg001-Ab002 |
Recombinant Bartonella henselae P26 antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Bartonella henselae antibodies in Bartonella henselae level test of animal Cat/Feline infectious disease with Cat scratch disease (CSD). |
||
Cat/Feline |
Chlamydophila felis |
CF0218 |
inflammation of feline conjunctiva, rhinitis and respiratory problems |
GMP-VT-P033-Tg001-Ag001 |
Anti-Chlamydophila felis CF0218 antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Chlamydophila felis level test of animal Cat/Feline infectious disease with inflammation of feline conjunctiva, rhinitis and respiratory problems. |
GMP-VT-P033-Tg001-Ab001/
GMP-VT-P033-Tg001-Ab002 |
Recombinant Chlamydophila felis CF0218 antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Chlamydophila felis antibodies in Chlamydophila felis level test of animal Cat/Feline infectious disease with inflammation of feline conjunctiva, rhinitis and respiratory problems. |
||
Cat/Feline, Dog/Canine |
Cryptococcus neoformans |
Hsp70 |
Cryptococcosis |
GMP-VT-P034-Tg001-Ag001 |
Anti-Cryptococcus neoformans Hsp70 antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Cryptococcus neoformans level test of animal Cat/Feline, Dog/Canine infectious disease with Cryptococcosis. |
GMP-VT-P034-Tg001-Ab001/
GMP-VT-P034-Tg001-Ab002 |
Recombinant Cryptococcus neoformans Hsp70 antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Cryptococcus neoformans antibodies in Cryptococcus neoformans level test of animal Cat/Feline, Dog/Canine infectious disease with Cryptococcosis. |
||
Rabbit, Hare, Rodent |
Francisella tularensis |
Outer Membrane Protein A (FopA) |
rabbit fever |
GMP-VT-P035-Tg001-Ag001 |
Anti-Francisella tularensis Outer Membrane Protein A (FopA) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Francisella tularensis level test of animal Rabbit, Hare, Rodent infectious disease with rabbit fever. |
GMP-VT-P035-Tg001-Ab001/
GMP-VT-P035-Tg001-Ab002 |
Recombinant Francisella tularensis Outer Membrane Protein A (FopA) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Francisella tularensis antibodies in Francisella tularensis level test of animal Rabbit, Hare, Rodent infectious disease with rabbit fever. |
||
Dog/Canine |
Rickettsia conorii |
OmpA and OmpB |
Mediterranean Spotted Fever or Boutonneuse Fever |
GMP-VT-P036-Tg001-Ag001 |
Anti-Rickettsia conorii OmpA and OmpB antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Rickettsia conorii level test of animal Dog/Canine infectious disease with Mediterranean Spotted Fever or Boutonneuse Fever. |
GMP-VT-P036-Tg001-Ab001/
GMP-VT-P036-Tg001-Ab002 |
Recombinant Rickettsia conorii OmpA and OmpB antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Rickettsia conorii antibodies in Rickettsia conorii level test of animal Dog/Canine infectious disease with Mediterranean Spotted Fever or Boutonneuse Fever. |
||
Dog/Canine |
Rickettsia massiliae |
TILS |
spotted fever group rickettsiae |
GMP-VT-P037-Tg001-Ag001 |
Anti-Rickettsia massiliae TILS antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Rickettsia massiliae level test of animal Dog/Canine infectious disease with spotted fever group rickettsiae. |
GMP-VT-P037-Tg001-Ab001/
GMP-VT-P037-Tg001-Ab002 |
Recombinant Rickettsia massiliae TILS antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other anti-Rickettsia massiliae antibodies in Rickettsia massiliae level test of animal Dog/Canine infectious disease with spotted fever group rickettsiae. |
Ruminant
Ruminants continue to be important in their traditional role in agricultural. In addition, ruminants play a vital role in the economy of poor, deprived, backward classes, and landless labors of developing countries. To make this small ruminant-based economy viable and sustainable, development of techniques for early and accurate diagnosis holds prime importance. However, ruminants suffer from numerous diseases, namely, fowl cholera, atrophic rhinitis, transmissible spongiform encephalopathies (TSEs), abortion, akabane disease, bluetongue disease, borna disease, borrelia theileri infection, botulism, bovine amyloidosis, bovine besnoitiosis, bovine Parainfluenza-3 Virus (BPI3) infection, bovine respiratory syncytial virus infection, bovine spongiform encephalopathy, bovine viral diarrhea, calf enteritis, enzootic pneumonia complex, winter dysentery, campylobacteriosis, caprine arthritis encephalitis, caprine pleuropneumonia, chlamydiosis, traveler’s diarrhea, chronic wasting disease, congenital diseases, contagious agalactia, contagious bovine pleuropneumonia, cystic hydatidosis, cysticercosis, enzootic bovine leukosis, fascioliasis, gastroenteritis, glanders, hemorrhagic septicemia, hormone disorders, hypodermiasis, immune dysfunction, leptospirosis, lumpy skin disease, maedi visna virus infection, mastitis, arthritis, pneumonia, nonbacterial diarrheic disease, paratuberculosis, paratuberculosis, PESTE des petits ruminants, pneumonia, prion associated diseases, Q fever, reproductive disease, respiratory disease, rift valley fever, rinderpest or cattle plague, rotaviral diarrhea, salmonellosis, schmallenberg disease, septicemia, septicemic colibacillosis, sexually transmitted infectious disease, trichomoniasis, tuberculosis/leprosy and so on. In such scenario, the rapid and specific detection of antigens and antibodies of these pathogens are developed by Genemedi. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for bovines/cattle infectious and non-infectious disease testing
Cattle diseases cost millions of money losses every year. In addition to death, they cause loss of production and frequently a loss of body condition. Unhealthy animals require more food and take longer time for growth than healthy ones. Generally, animals are born free of diseases or parasites. But they usually acquire these diseases either through contact with diseased animals or due to improper sanitation, feeding, care and management. Knowledge of cattle diseases is necessary from public health point of view also as many diseases can be transmitted to man through milk. Keeping animals healthy by employing sound principles of sanitation, management and feeding and by judicious use of appropriate and dependable vaccines are the practical and economical ways to avoid losses from the disease. By proper management and feeding, the dairy farmer can, to a great extent, prevent disease out-breaks. Cattle are infected by a community of endemic pathogens with different epidemiological properties that invoke different managerial and governmental responses. Genemedi developed the antigen and antibody to detect the abortion, acute severe metritis, borna disease, borrelia theileri infection, bovine amyloidosis, bovine besnoitiosis, bovine parainfluenza-3 virus (bpi3) infection, bovine respiratory syncytial virus infection, bovine spongiform encephalopathy, bovine viral diarrhea, calf enteritis, chronic endometritis, chronic wasting disease, contagious bovine pleuropneumonia, cystic hydatidosis, cysticercosis, diarrhea, enzootic bovine leukosis, enzootic pneumonia complex, gastrointestinal infections, hormone disorders, hypodermiasis, infectious bovine rhinotracheitis, infectious pustular vulvovaginitis, ketosis, mastitis, arthritis and pneumonia, milk fever, paratuberculosis, respiratory and enteric infections, respiratory syndrome, retained placenta, rotaviral diarrhea, septicemia, severe mastitis, systemic infection in neonates, thromboembolic meningoencepahlitis (teme), transmissible spongiform encephalopathies (tses), tuberculosis/leprosy and so on.
Due to the physiology and structure of bovines, cattle health issues are also unique. With their four-chambered stomachs and a surprising susceptibility to heat, cattle require special care, monitoring and handling to maintain optimum health and longevity. Good grassland management will ensure that cattle have access to plenty of leafy grass that they can readily digest and will provide all of their energy requirements for maintenance and growth. In addition, the diet must also provide small amounts of certain essential chemical elements (trace elements). Deficiencies of any of these micronutrients can result in ill-thrift. Fortunately, there are a few simple ways to improve your herd’s health. Watchful ranchers can detect early signs of the most common cattle health issues, and then take preventative measures to correct potentially detrimental conditions. Genemedi developed the antigen and antibody to detect the non-infectious disease such as immune dysfunction, abortion and teratology, nonbacterial diarrheic disease, Prion associated diseases (Scrapie, Bovine Spongiform Encephalopathy, Chronical Waste Disease), winter dysentery and so on.
Diagnostic antibodies and antigens for Ovines/Sheep disease testing
A sound management program to keep animals healthy is basic to production of both sheep and goats. Producers must observe animals closely to keep individual animals and the whole herd or flock healthy and productive. To recognize clinical signs of diseases common to sheep and goats, it is important to be familiar with what is normal. Producers should assess the herd or flock’s general health on a regular basis, including vital signs, body condition, and coat. Correct diagnosis of sheep is most difficult. Hence, Genemedi developed the antigen and antibody to detect the disease such as campylobacteriosis, caprine arthritis encephalitis, caprine pleuropneumonia, congenital diseases, contagious agalactia, gastroenteritis, leptospirosis, lumpy skin disease, sheeppox and goatpox, maedi visna virus infection, mycoplasma pneumonia, PESTE des petits ruminants, pneumonia, rift valley fever, sexually transmitted infectious disease and so on.
Diagnostic antibodies and antigens for Caprine/Goat disease testing
Goats are important domestic animals in many parts of the world. They provide substance in the form of food and clothing. The rising demand for goat meat, milk, and cheese offers commercial goat production opportunities. Goats are also a source of immediate income. The management of goat health is a critical aspect for improving goat production. It is important to understand health and disease of goat. Hence, Genemedi developed the antigen and antibody to detect the disease such as abortions and mortality in neonates, akabane disease, bacteremia, cholangitis, bluetongue disease, caprine pleuropneumonia, chlamydiosis, cholecystitis, congenital diseases, contagious agalactia, gastroenteritis, leptospirosis, maedi visna virus infection, paratuberculosis, Q fever, respiratory syndrome, rift valley fever, schmallenberg disease, septicemic colibacillosis, traveler’s diarrhea, trichomoniasis, urinary tract infection (UTI) and so on.
Diagnostic antibodies and antigens for deer disease testing
Managing the deer population is essential to maintaining or improving forest health. It plays a crucial role in the ecosystem, providing food for large predators such as gray wolves (Canis lupis), cougars (Puma concolor), bobcats (Lynx rufus), and coyotes (Canis latrans). They feed primarily on grasses, herbaceous plants, fruits, and legumes and are active throughout the year. Their economic importance includes the use of their meat as venison, their skins as soft, strong buckskin, and their antlers as handles for knives. Dears are prone to diseases caused by a long list of environmental pathogens. Those can rapidly spread throughout deer populations and cause outbreaks that cause death and chronic illness. Hence, Genemedi developed the antigen and antibody to detect the disease such as cholecystitis, bacteremia, cholangitis, urinary tract infection (UTI), traveler’s diarrhea, septicemic colibacillosis, respiratory syndrome and so on.
Companion Animal
The pet animals such as dogs and cats are susceptible to many diseases including personnel illnesses and infection. Infectious diseases are transmitted by contact with infected dogs or cats or wildlife. Disease causing agents (pathogens) include parasites, viruses, bacteria, fungi, and protozoa. Hundreds of these infectious pathogens have the potential to be transmitted between dogs, cats and other animals; however, only a subset of these commonly causes problems. Additionally, some of these pathogens can be spread between pets and people (termed “zoonotic”), causing illness in people adding additional concerns. When a case of infectious disease is suspected, it is important to get a diagnosis quickly and identify susceptible animals that might have been exposed. The diagnosis of pet disease is complex because the spectrum of clinical signs and clinicopathological abnormalities is broad and often nonspecific. However, Genemedi developed the antigen and antibody to detect the various disease of pets including dogs, cat, rabbit and so on. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for dog/canine infectious and non-infectious disease testing
Dogs are the best friends, and most human keep pets for its ompanionship. Dogs need a proper space, diet and health care for their healthy life. However, sometimes the dogs are affected by the different infectious diseases such as amyloidosis, anaplasmosis, anemia, intestinal infection, canine cyclic thrombocytopenia, canine herpes, canine infectious tracheobronchitis, canine parvovirus infection, conjunctivitis, croup, dirofilariasis, gastroenteritis, hepatitis, hormone disorders, inflammation, leptospirosis, mediterranean spotted fever, otitis media, pancreatitis, pharyngitis, pneumonia, pregnancy, rabies, rocky mountain spotted fever, spotted fever group rickettsia, tracheobronchitis, visceral leishmaniasis and so on. Genemedi developed the antigen and antibody to detect these diseases at early stages.
Dog is known to be a very intelligent animal that can detect human psychology easily, and even a well-trained dog can be used as a substitute of a qualified person to perform certain specific crucial functions. Further, dogs play a pivotal role to detect crimes and criminals. The common diseases of dogs can be categorized into infectious, non-infectious, and non-specific diseases. Among non-infectious diseases, dogs are mostly prone to fracture, lacerated wound, abortion, dystocia, Cancer, Diabetes and vitamin deficiency and the most frequent non-specific cases are gastroenteritis, otorrhea, dermatitis, and alopecia. A good knowledge of the epidemiology of these diseases of dogs is important for their prevention and control. Genemedi developed the antigen and antibody to detect the various non-infectious disease of dogs.
Diagnostic antibodies and antigens for cat/feline infectious and non-infectious disease testing
Cats are credited with promoting socialization among older individuals and physically or mentally disabled people. Research has shown that cats can provide emotional support, improve moods, and contribute to the overall morale of their owners. Although cats are great companions, cat owners should be aware that sometimes cats can carry harmful germs that can cause a variety of illnesses. Since cats are fiercely independent and sometimes mysterious little creatures, it can be difficult for pet parents to tell the difference between a minor issue and a serious health problem. Genemedi developed the antigen and antibody to detect the amyloidosis, anaplasmosis, canine angiostrongylosis, canine brucellosis, cardiac disease, cat HIV, cat scratch disease, feline infectious enteritis, feline infectious peritonitis, feline panleukopenia, feline viral rhinotracheitis, hormone disorders, inflammation of feline conjunctiva, leishmaniasis, leukemia, pancreatitis, respiratory disease, rhinitis and so on.
No one likes to think about their cat getting sick or contracting a disease, but unfortunately there are many diseases affect the cat. It is important to understand signs and symptoms of cat and most importantly to prevent cat diseases. Genemedi developed the antigen and antibody to detect the non-infectious disease such as chronic kidney disease, degenerative joint disease (arthritis), diabetes, hypertension (high blood pressure), hyperthyroidism lower urinary tract disease, obesity and so on.
Diagnostic antibodies and antigens for rabbit infectious disease and non-infectious testing
Rabbits are perhaps the most popular small mammals kept as pets. They make great companions and can live a dozen or more years when they are cared for properly. However, they do commonly develop a few illnesses and infection that all rabbit owners should be aware to prevent the disease. The most common diseases of rabbits include rabbit hemorrhagic disease and so on have been detected using the antigen and antibody produced by Genemedi.
It’s important to know how to care and prevent common types of rabbit illness. Rabbits fed with a suitable diet and kept in a healthy environment can live as long as 10 to 12 years. The most common diseases of rabbits include digestive system problems, respiratory infections, and skin disorders. In addition to the infectious disease testing, Genemedi also developed antigen and antibody to detect the common rabbit illness.
Swine
Diagnostic antibodies and antigens for swine disease testing
Pork accounts for more than one-third of meat produced worldwide and is an important component of global food security, agricultural economies, and trade. Infectious diseases are among the primary constraints to swine production, and the globalization of the swine industry has contributed to the emergence and spread of pathogens. Infectious diseases result in direct losses to livestock production through mortality, loss of productivity, trade restrictions, reduced market value, and often food insecurity. The constant threat of endemic and emerging diseases affecting swine, which in some instances also impact human health, highlight the potential vulnerability of pork production around the world. Indeed, infectious diseases of swine are among the primary constraints to pork production and trade. Genemedi developed the antigen and antibody to detect the swine disease such as african swine fever, arthritis, aujeszky’s disease, bacteremia, brucella suis, chlamydial infections, cholangitis, cholecystitis, classical swine fever, diarrhea, ecephalitis, endocarditis, enzootic pneumonia, erysipelas, gastroenteritis, glässers disease, idiopathic vesicular disease, japanese encephalitis, leptospirosis, meningitis, multisystemic wasting syndrome, nipah virus infection, porcine colonic spirochetosis, porcine diarrhea, porcine enzootic pneumonia, porcine pleuropneumonia, porcine reproductive and respiratory syndrome, proliferative hemorrhagic enteritis, pseudorabies, reproductive disease, respiratory disease, salmonellosis, septicemia, swine brucellosis, swine enteric coronavirus disease (SECD), swine flu, swine pleuropneumonia, swine vesicular disease, traveler’s diarrhea, urinary tract infection, viral enteritis, white chick syndrome and so on. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Equine
Diagnostic antibodies and antigens for infectious and non-infectious equine/horse disease testing
Common health problems and infections are a constant threat to the health and welfare of horses. Several diseases have assumed greater importance as the performance and pleasure horse populations and equine activities have increased and there are new owners who do not understand the implications of equine infectious disease outbreaks to their animals. In addition, emerging diseases have beset the equine species in recent years. Genemedi developed the antigen and antibody to detect the horse herpes myeloencephalopathy, respiratory disease, horse infectious anemia, equine influenza, thrombocytopenia, anaplasmosis, contagious equine metritis, horse viral arteritis, horse piroplasmosis, horse theileriosis, pneumonia; urinary tract infections, respiratory system infections, dermatitis, soft tissue infections, bacteremia, bone and joint infections, gastrointestinal infections, eastern equine/horse encephalitis (EEE), equine/horse morbillivirus pneumonia and so on. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Most horses are very resilient animals, yet like all animals, they are subject to their own set of illnesses. They are susceptible to a number of common horse health problems and issues ranging from illness and disease, to injuries. Horse owners are responsible for the supervision of horses should be able to recognize signs of ill health. It is also important that owners/keepers to diagnose or treat any illness, injury or disease. Hence, Genemedi developed the antigen and antibody to detect the Arthritis/Osteoarthritis, laminitis, Equine Cushing’s Disease and so on.
Avian
Diagnostic antibodies and antigens for avian disease testing
Infectious diseases continue to threaten the sustainability, productivity and growth of the poultry industry worldwide and some present a risk to public health. Many are also present in wild bird populations, with the potential to spill over into domestic birds. The avian infectious disease cause huge economic loss in poultry production and are of great significance in public health. However, they are usually not covered in the systems for reporting of animal diseases. Several methods have been employed to develop conventional assays to detect avian disease, Genemedi developed the antigen and antibody to detect the upper respiratory tract infection, newcastle disease, duck hepatitis, derzsy’s disease, infectious bursal disease, chlamydiosis, respiratory disease, arthritis, duck plague, avian influenza, cholecystitis, bacteremia, cholangitis, urinary tract infection (UTI), and traveler’s diarrhea, respiratory disease, avian metapneumovirus infection, treponeme, encephalomyelitis, infectious bronchitis, mycoplasmosis, nephritis, blackhead disease (histomoniasis), bordetellosis, campylobacteriosis, infectious anemia, chlamydia, duck septicaemia, Goose ‘flu, riemerellosis, polyserositis, egg drop syndrome, erysipeloid, fowl cholera, fowl typhoid, pullorum, fowlpox, gastroenteritis, hemorrhagic nephritis, enteritis of geese, hepatitis-splenomegaly syndrome, immunosuppression, inclusion body hepatitis, infectious bronchitis, infectious coryza, infectious laryngotracheitis, infectious synovitis, avian mycoplasmosis, infectious sinusitis, mycoplasma arthritis, lymphoid leukosis, Marek’s disease, mycoplasma air sacculitis, mycoplasmosis, ornithobacteriosis, feather disease, psittacosi, pullorum, reticuloendotheliosis, salmonellosis, turkey hepatitis, swollen head syndrome, white chick syndrome, young pigeon disease syndrome and so on. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Fish
Diagnostic antibodies and antigens for fish disease testing
Fishes are susceptible to diseases caused by a large number of infectious agents including viruses, bacteria, true fungi, fungal-like microrganisms, other protists, and metazoans. The major impact is on farms that rear young fish where cumulative mortality can reach 90–100%. In addition to direct losses due to mortality, the disease has a negative impact on the breeding of endangered fish stocks, causes restrictions on the movement of infected fish or survivors, and so mortality decreased fish production levels and deformities that occur in the survivors. Therefore, identification of pathogens, prevention and control strategies are important to reduce diseases that causes damage of aquaculture production. Genemedi developed the antigen and antibody to detect the koi herpesvirus disease, infectious salmon anemia, infectious pancreatic necrosis, spring viraemia of carp, viral hemorrhagic septicemia, infectious hematopoietic necrosis, rift valley fever and so on. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Multiple Species
Diagnostic antibodies and antigens for multiple animal disease testing
Animal disease outbreaks have been shown to cause major economic losses over the centuries. Several diseases such as walking pneumonia, anaplasmosis, aspergilloma, athlete’s foot, babesiosis, bacteremia, brucellosis, campylobacteriosis, candidemia, candidiasis, cellulitis, cholangitis, cholecystitis, clostridial enterotoxicosis, clostridiosis, colitis, cryptosporidiosis, dermatophytosis, diarrhoea, endocarditis, epizootic hemorrhagic disease, esophagitis, foot and mouth disease, fungal infection of nail, jock itch, and ringworm, fungemia, gastroenteritis, giardiasis, hemolytic-uremic syndrome, haemorrhage, hepatitis E, intra-abdominal infection, meningitis, minor skin infections, neonatal meningitis, neosporosis, osteomyelitis, pelvic inflammatory diseases, peritonitis, pneumonia, prostatitis, pulmonary infections, sepsis, septicemia, skin infections, tinea or ringworm, toxic shock syndrome, toxoplasmosis, traveler’s diarrhea, trichinosis, tuberculosis, typhoid, urinary and septic infections, west nile fever, wound infection and so on affect multiple animals. An early detection system enables the timely detection and identification of an incursion or emergence/re-emergence of a disease/infection in a given country, zone or compartment prevent the disease outbreak. The exquisite specificity of antigen-antibody interactions has led to the development of a variety of immunologic assays, which can be used to detect the presence of either antibody or antigen. Genemedi developed the antigen and antibody to detect the animal diseases. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Abstract - Animal health diagnosis
Animal infectious diseases pose a continuing threat to animal health, food safety, national economy, and the environment. Zoonotic infections, also named as zoonoses, involve veterinary pathogens that are sustained in animal populations but can be transmitted to and cause disease in humans. In the event of veterinary outbreaks, it is essential to make rapid and accurate diagnosis to control and prevent the spread of diseases. Here we discuss different diagnostic methods available to identify animal diseases and zoonotic infections. Efficient diagnosis strategies are critical for controlling and eliminating animal diseases and zoonoses, further protecting and improving animal health, quality, and productivity.
Introduction of animal infectious disease
Animal diseases are globally important diseases and lead to huge economic losses. The emergence of animal disease infections and their worldwide distribution are predisposed by climate change, intense livestock production, illegal movements of animals and humans, regional civil wars and increasing trade.
The spread of infectious diseases has increased the risk of catastrophic animal losses. Some animal diseases can transmit from animals to humans and vice versa, termed zoonoses. Zoonoses encompass some of the most ancient communicable diseases, such as rabies and plague, as well as newly recognized emerging infections, such as hantavirus pulmonary syndrome (HPS) and severe acute respiratory syndrome (SARS). Routes of transmission of zoonosis to humans through direct contact or through food, water, or the environment, contributing to 61% of infectious organisms affecting humans [1, 2].
Zoonosis can be caused by veterinary pathogens, including bacteria, fungi, mycobacteria, parasites, viruses, and prions. The disease symptoms in humans range from mild and self-limiting to fatal [3]. Table 1 highlights some animal infectious diseases and their veterinary pathogens, in Table 2 we highlight some selected important zoonotic diseases and their veterinary pathogens.
| Avian | Avian influenza | Avian influenza virus | Anti-Avian Influenza Virus NP mouse monoclonal antibody (mAb) Anti-Avian Influenza Virus Haemagglutinin (HA) mouse monoclonal antibody (mAb) |
| Canine | Rabies | Rabies virus (RV) | Anti-Rabies virus Glycoprotein NP mouse monoclonal antibody (mAb) |
| Ruminants | Brucella abortus, Brucella melitensis, Brucellosis | Brucella abortus, Brucella melitensis (Brucellosis) | Anti-Brucella abortus/Brucella melitensis Omp2b mouse monoclonal antibody (mAb) Anti-Brucella abortus/Brucella melitensis P17 mouse monoclonal antibody (mAb) Anti-Brucella abortus/Brucella melitensis BP26 mouse monoclonal antibody (mAb) Anti-Brucella abortus/Brucella melitensis virB5 mouse monoclonal antibody (mAb) Anti-Brucella abortus/Brucella melitensis OMP28 mouse monoclonal antibody (mAb) Anti-Brucella abortus/Brucella melitensis VirB12 mouse monoclonal antibody (mAb) |
| Ruminants | Bovine spongiform encephalopathy (BSE) | Bovine spongiform encephalopathy (BSE)/prion | Anti-prion PrP mouse monoclonal antibody (mAb) Anti-prion PrPSc mouse monoclonal antibody (mAb) Anti-prion PrPRes mouse monoclonal antibody (mAb) |
| Unknown (possibly bats) | Ebola Hemorrhagic Fever | Ebola | |
| Rodents, cattle | Monkeypox, cowpox | Orthopoxviruses | |
| Rodents | Hantavirus pulmonary syndrome, hemorrhagic fever with renal syndrome, hantaviral illness | Hantaviruses, Bunyavirus | |
| Rodents | Lymphocytic choriomeningitis virus, Bolivian (Machupo), Brazilian (Sabia), Argentine (Junin), African (Lassa) hemorrhagic fevers | Arenaviruses | |
| Rodents | Plague | Yersinia pestis | |
| Livestock | Q fever | Coxiella | Anti-Coxiella com1 mouse monoclonal antibody (mAb) |
| Birds, mammals, reptiles, amphibians | Salmonellosis | Salmonella spp | Anti-Salmonella spp mouse monoclonal antibody (mAb) |
| Wild and domestic animals | Leptospirosis | Leptospira interrogans (multiple serovars) | |
| Rabbits, hares, voles, muskrat, beaver, rodents | Tularemia | Francisella tularensis (var tularensis and palaeartica) | Anti-Salmonella spp mouse monoclonal antibody (mAb) |
| Livestock, wild ruminants | Hemolytic uremic syndrome/E. coli infection | Escherichia coli O157:H7 | Anti-Escherichia coli O157:H7 OmpC mouse monoclonal antibody (mAb) Anti-Escherichia coli O157:H7 Intimin γ1 mouse monoclonal antibody (mAb) |
| Birds | Psittacosis | Chlamydia psittaci | Anti-Chlamydia psittaci MOMP mouse monoclonal antibody (mAb) |
| Equine | Glanders | Burkholderia mallei | Anti-Burkholderia mallei Hcp1 mouse monoclonal antibody (mAb) |
| Cats | Cat scratch disease | Bartonella, henselae/quintana | Anti-Bartonella henselae P26 mouse monoclonal antibody (mAb) |
| Livestock | Anthrax | Bacillus anthracis | |
| Wild and domestic animals | Cryptosporidiosis | Cryptosporidium parvum | Anti-Cryptosporidium parvum P23 mouse monoclonal antibody (mAb) |
| Wild and domestic animals | Giardiasis | Giardia lamblia | Anti-Giardia lamblia α1-giardin mouse monoclonal antibody (mAb) |
| Felids | Toxoplasmosis | Toxoplasma gondii | Anti-Toxoplasma gondii P30 mouse monoclonal antibody (mAb) |
| Dogs, cats, raccoons | Larval migrans | Toxocara canis, T. cati, Baylisascaris procyonis | |
| Dogs, cats, raccoons | Cutaneous larval migrans | Ancylostoma spp., Strongyloides spp. | |
| Swine, rodents, wild carnivores | Trichinosis | Trichinella spp. | Anti-Trichinella cystatin-like protein mouse monoclonal antibody (mAb) |
| Mammals, some birds | Dermatophytosis (ringworm) | Microsporum canis, Trichophyton |
The strategies used in diagnosis of animal infectious disease for animal health
Rapid diagnosis is critical for the implementation of efficient control strategies against animal infectious and zoonotic diseases. Developing high-quality diagnostic methods and understanding animal diseases infection dynamics are important to obtain reliable diagnostic results. Here we discuss some of the most common animal disease and zoonotic disease identification methods, currently being used both in experimental and diagnostic assays.
1. Lateral flow assays
Lateral flow test, a simple cellulose-based device developed to detect the presence of a target analyte in a liquid sample [4]. There are two main variations of lateral flow tests: antigen-based test, using monoclonal antibodies to detect the specific viral antigens, another is antibody-based test, using viral antigen protein to measure the specific antibody level in a sample. For antigen-based test, test strips are coated with antibodies that bind to a viral protein and if the animal’s sample contains such proteins, they will bind to the antibodies, to form a colored indicator on the strip. Samples can be feces, eye mucus, whole blood, serum or plasma. For antibody-based test, test strips are coated with viral antigens that bind to antibodies and if the animal blood sample contains such antibodies, they will bind to the viral antigens, to form a colored indicator on the strip. Samples can be whole blood, serum or plasma [5].
Colloidal gold nanoparticles are the most widely adopted material to induce a color change when it comes in contact with the analyte. Based on the specific immune response of antigen and antibody, colloidal gold particles were used as one of the tracer markers. Driven by solvent chromatography, the markers had an immune response on the C/T line, and the detection results could be obtained according to the color of the T line. GICA samples can be whole blood, serum or plasma, and studies have shown that the colloidal gold reagent has a high consistency in detecting whole blood, plasma or serum [6]. The test results could be provided between 10-30 mins from sample collection.
Due to the low sensitivity of this test, it would effectively work only on symptomatic individuals and these tests could be less reliable in comparison with RT-PCR tests. However, it could be quickly performed at the point-of-care, or in community settings without the need for expensive equipment. An overview of the process for lateral flow assay is presented in Figure 1.
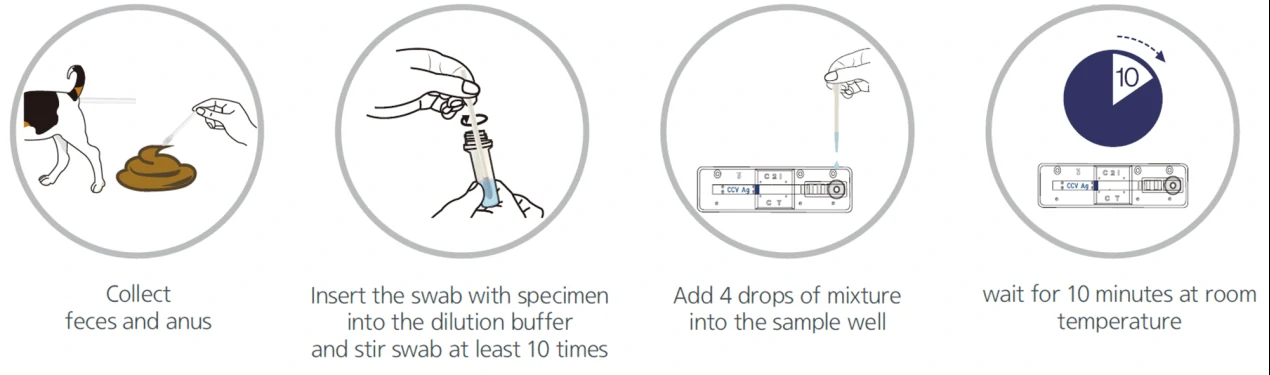
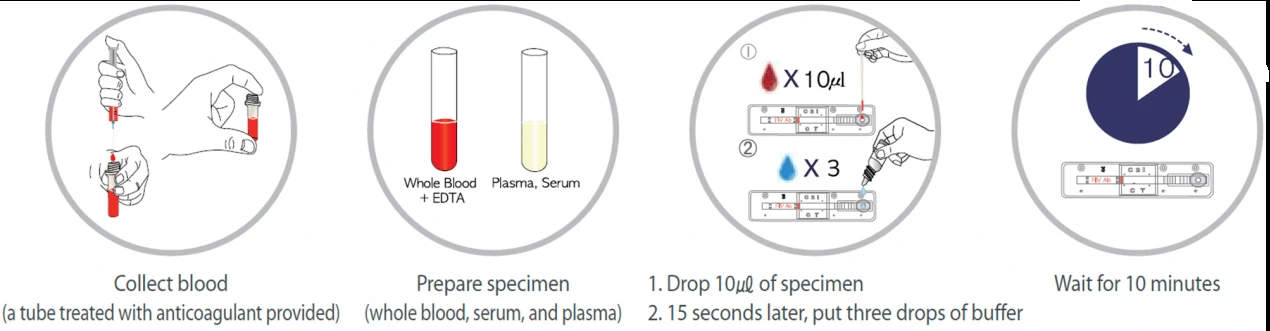
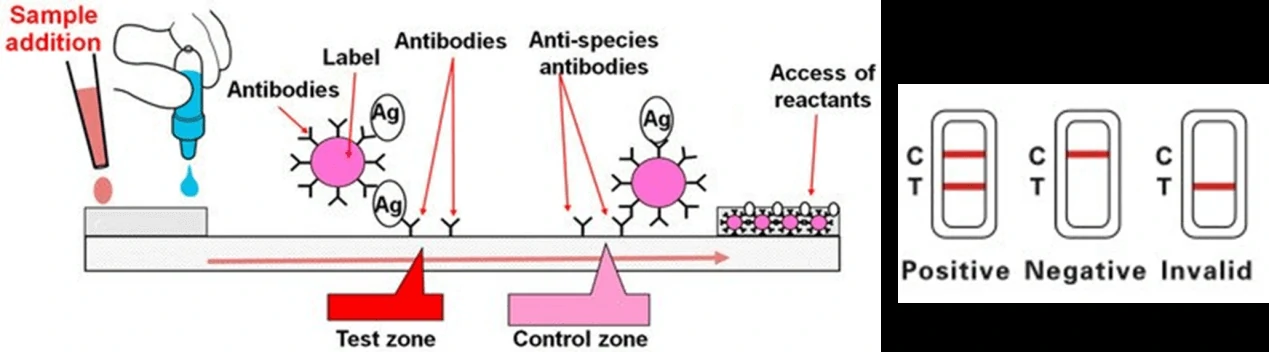
2. Enzyme-Linked Immunosorbent Assays (ELISA)
Enzyme-linked immunosorbent assays (ELISAs) incorporate the sensitivity of simple enzyme assays with the specificity of antibodies, by employing antigens or antibodies coupled to an easily-assayed enzyme. As such ELISA is much more rapid method than immunoblotting to detect specific viral protein from a cell, tissue, organ, or body fluid. There are two main variations of ELISAs: antigen-capture ELISA (detecting viral proteins), involve attachment of a capture antibody to a solid matrix for the viral protein of interest, while antibody-capture ELISA measures the specific antibody level in a sample, by coating viral antigen protein on a solid surface.
There are two principles based on antigen-capture and antibody-capture ELISAs. In a general, ELISAs are considered a highly sensitive method that can detect a fairly low number of proteins at the range of picomolar to nanomolar range (10-12 to 10-9 moles per liter). ELISA method was found useful as a diagnostic tool to detect influenza viral antigen much quicker than other conventional virus detection methods [7]. In another previous study, comparison of ELISA, with conventional methods has demonstrated ELISA superiority for the rapid detection and identification of influenza A virus [8]. A simplified and standardized neutralization enzyme immunoassay (Nt-EIA) was developed to detect measles virus growth in Vero cells and to quantify measles neutralizing antibody [9].
Newer EIA formats for hepatitis C virus diagnostics have been constantly evaluated [10, 11]. As such ELISAs are being used for plethora of application both in experimental and diagnostic virology including dengue, and influenza [12-14]. On the other hand, although rapid than traditional plaque assays or TCID50, ELISA assays sometimes could be quite expensive, due to the cost of reagents used. Unfortunately, sometimes required antibodies may not be commercially developed as well. In contrast, attempts to develop antibodies in-house may be quite expensive. Additional variability may also be introduced due to high background signals generated by non-specific binding, or cross-reactivity with non-viral protein targets.
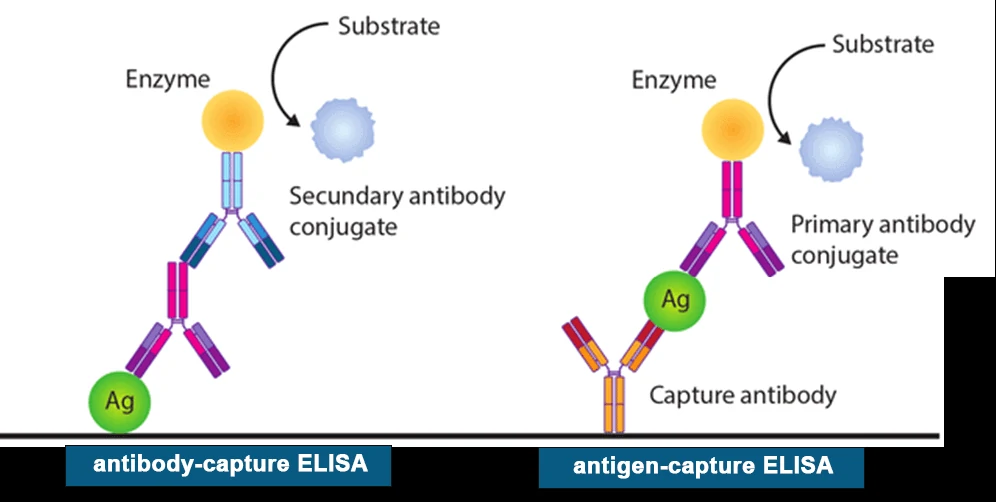
3. Immunofluorescence staining (IF)
The immunofluorescence staining is to detect viral antigen using virus-specific monoclonal or polyclonal antibodies fluorescent staining of viral antigens is visualized under a fluorescent microscope.
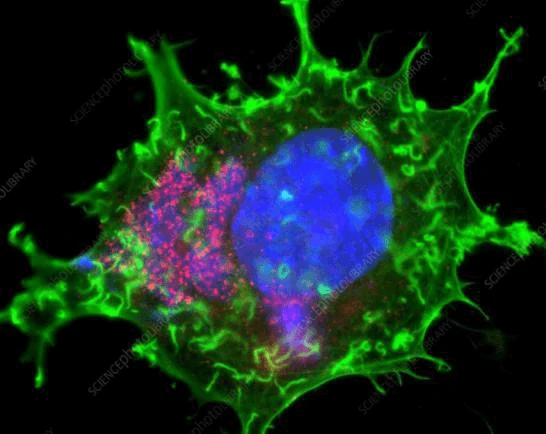
4. Immunohistochemistry (IHC)
Immunohistochemistry is to detect viral antigen in formalin-fixed paraffin-embedded tissues using virus-specific monoclonal or polyclonal antibodies followed by an enzyme-linked secondary antibody and chemical substrate; IHC can be visualized under alight microscope.
5. In situ hybridization (ISH)
In situ hybridization (ISH) is to detect viral nucleic acid present in fixed tissues using a labeled complementary DNA, RNA or modified nucleic acid strand. Different with PCR approach where viral nucleic acid in a sample is amplified before detection, ISH detects viral nucleic acid that is not going through an amplification process.
6. Virus isolation (VI)
Obtaining the virus isolate that can efficiently grow in cell culture is critical for pathogenesis study, development of diagnostic assays, and vaccine development. However, viral culture results do not yield timely results to inform clinical management. Shell-vial tissue culture results may take 1-3 days, while traditional tissue-cell viral culture results may take 3-10 days. Due to the long incubation time, high technical requirements, and must be carried out in a level III safe biological laboratory, it is not suitable for rapid virus diagnosis during the epidemic period [17].
7. Electron microscopy (EM)
Electron microscopy allows direct visualization of virus particles. Two EM techniques are commonly used in diagnostic laboratories: negative-stain EM and ultrathin-section EM. Negative-stain EM for detection of virus particles in a fluid matrix; ultrathin-section EM for detection of virus particles in fixed tissues or cells. Based on characteristic morphology and size of virus particles observed under EM, viruses can be assigned to appropriate family, e.g., coronavirus-like particles were observed in some feces during initial investigation of diarrheic cases caused by porcine delta-coronavirus. Although EM cannot identify viruses to the species level, identification to the family level can still facilitate next-step testing to achieve definite diagnosis. However, EM generally is less sensitive and needs presence of sufficient amount of virus (about105–6virions per milliliter) in examined specimens. In addition, EM requires expensive equipment and highly skilled microscopist.
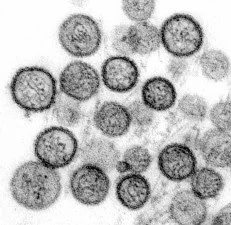
8. Molecular Methods
The development of molecular methods for the direct identification of a specific viral genome from the clinical sample is one of the greatest achievements of the 21st century. Polymerase chain reaction (PCR) is a technique that can in vitro amplify specific nucleic acid sequences and produce billion copies of target sequences within a few hours. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) is proven technology leaders for rapid detection and molecular identification for most known human viruses [19]. Virus can be detected through real-time RT-PCR with primers against two segments of virus RNA genome. However, high mutation rates may lead to extensive changes in viral nucleic acid sequences making dedicated PCR primer use irrelevant, therefore there is high demand for the development of rapid and universal virus identification and detection technologies.

Summary
In the recent years, importance of animal disease and their public health effects have been well recognized worldwide. Animal disease, more significantly, zoonotic disease cause human mortality and morbidity, and also affect livestock’s production, decrease availability of food and create barriers for international trade. Rapid diagnosis is critical for the implementation of efficient control strategies against animal disease and zoonotic disease. Understanding animal disease infection dynamics and collecting appropriate specimens at the appropriate time window are also important to obtain reliable diagnostic results. A number of virological and serological methods have been developed and used for animal disease diagnostic testing. RT-PCR is the method of common choice for the detection of animal disease; IHC combined with hematoxylin and eosin staining has also been commonly used to examine histopathological lesions caused by animal disease. Success rate of virus isolation in cell cultures has been low. Serological assays can provide information about previous exposure to animal disease and also determine antibody responses to infection or vaccination when vaccines are available. Rolling out serological test would be an effective strategy to determine the percentage of the population that is immune and have shown no symptoms for the animal disease. Thereby, determining the exact magnitude of the outbreak and enabling governments to assess containment strategies to slow down the spread. The major drawbacks with these immunoassays are their accuracy and sensitivity of the test results. Therefore, there needs to be extensive research and testing done to develop new cost-effective methods to quickly and easily determine animal disease infection. Whereas, any such emerging approach must be carefully evaluated for its efficiency, accuracy, and linear range. The FDA approval and evaluation of each diagnostic technique is necessary before it can be used in practice.




