Anti-small molecules (Chemicals, Antibiotics£¬Mycotoxins,etc.) antibodies and small molecules competitive antigens (Carrier-coupled antigen, immunogen, hapten-carrier conjugates, BSA-conjugated, OVA-conjugated), competitive ELISA validated
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

Competitive immunoassay-validated anti-Hapten (small molecules, chemicals) antibody against hapten (small molecules, chemicals) and hapten-carrier conjugated competitive antigens(Carrier-coupled antigen,immunogen,hapten-carrier conjugates, BSA-conjugated, OVA-conjugated).
The anti-Hapten antibodies against haptens had been validated with our hapten-carrier conjugates via competitive ELISA test.
GeneMedi offers paired anti-small molecules (Chemicals, Antibiotics,Mycotoxins,Hormones,Drugs of Abuse, etc.) antibodies (monoclonal antibody, mab) and small molecules competitive antigens (Carrier-coupled antigen, immunogen, hapten-carrier conjugates, BSA-conjugated, OVA-conjugated) for the rapid test and diagnostics kit of Growth Promoters, Toxic Heavy Metal, Myotoxins,Nutritions, Food Safety, Pesticides, Drugs of Abuse, Allergen, Antibiotics, Agricultural, Water Contamination and so on.
All our anti-chemicals antibodies and small molecules hapten-carrier conjugates antigens (BSA-conjugated, OVA-conjugated)are suitable for in competitive ELISA, Lateral flow immunoassay (LFIA) and other immunoassays in diagnostics and rapid test kit. The carrier-coupled antigens of small molecules can act as immunogens.
Trimethoprim is a synthetic derivative of trimethoxybenzyl-pyrimidine with antibacterial and antiprotozoal properties. Sulfamethoxazole with trimethoprim is a fixed antibiotic combination that is widely used for mild-to-moderate bacterial infections and as prophylaxis against opportunistic infections. Recently, our R&D department demonstrated that our GMP-SMT-73-Ab-1 (Anti-Trimethoprim(TMP) mouse monoclonal antibody) has a large linear range and good sensitivity against the GMP-SMT-73-Ag-1 (BSA-Trimethoprim(TMP)). Below is the result of GeneMedi’s Anti-Trimethoprim(TMP) mouse monoclonal antibody validation with BSA-Trimethoprim(TMP) in ELISA. We highly recommend the Ab&Ag to you.
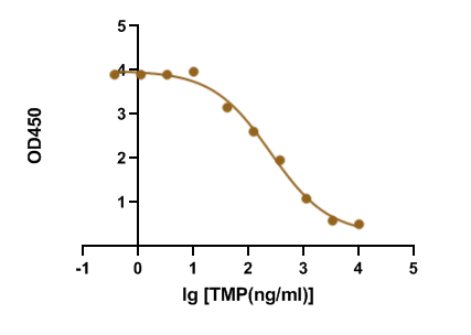
Genemedi supplies Small Molecule Diagnostic for a wide selection of choices (please see below).
Cat No. | Small molecules (Chemicals, Antibiotics, Mycotoxins, Hormones, Drugs of Abuse, etc.) | Carrier-coupled antigen/immunogen
(hapten-carrier conjugates) | Anti-Hapten Antibody | Bioactivity validation | ELISA IC50(ppb) | Order |
|---|---|---|---|---|---|---|
Neomycin(NM) |
BSA-Neomycin(NM); OVA-Neomycin(NM) |
Anti-Neomycin(NM) mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
0.50 |
||
Bisphenol A |
BSA-Bisphenol A; OVA-Bisphenol A |
Anti-Bisphenol A mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
2.00 |
||
Thyroxin T4 |
BSA-Thyroxin T4; OVA-Thyroxin T4 |
Anti-Thyroxin T4 mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
3.00 |
||
Forchlorfenuron |
BSA-Forchlorfenuron; OVA-Forchlorfenuron |
Anti-Forchlorfenuron mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
0.20 |
||
Norethisterone |
BSA-Norethisterone; OVA-Norethisterone |
Anti-Norethisterone mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
0.4-0.5 |
||
Flugestone Acetate |
BSA-Flugestone Acetate; OVA-Flugestone Acetate |
Anti-Flugestone Acetate mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
0.4-0.5 |
||
Thyroxin T3 |
BSA-Thyroxin T3; OVA-Thyroxin T3 |
Anti-Thyroxin T3 mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
0.4-0.5 |
||
IGFBP-1(Insulin-like growth factor binding protein 1) |
BSA-IGFBP-1(Insulin-like growth factor binding protein 1); OVA-IGFBP-1(Insulin-like growth factor binding protein 1) |
Anti-IGFBP-1(Insulin-like growth factor binding protein 1) mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
/ |
||
Tolfenamicacid |
BSA-Tolfenamicacid; OVA-Tolfenamicacid |
Anti-Tolfenamicacid mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
2.00 |
||
Carprofen |
BSA-Carprofen; OVA-Carprofen |
Anti-Carprofen mouse monoclonal antibody |
Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody;
Lateral flow immunoassay (LFIA); |
1.00 |
Cat No. | Species | Biomarker | Cat No.of Antigen | Bioactivity validation of Antigen | Cat No.of Antibodies | Bioactivity validation of Antibodies | Order |
|---|---|---|---|---|---|---|---|
Human |
acetaminophen (Paracetamol) |
GMP-SMT-ParacetamolAg01:BSA-acetaminophen (Paracetamol) ;GMP-SMT-ParacetamolAg02: OVA-acetaminophen (Paracetamol) |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-acetaminophen (Paracetamol), acetaminophen (Paracetamol) antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in Paracetamol level test of Pain (hepatotoxicity) and related syndrome evaluation. |
GMP-SMT-Paracetamol-Ab01 Anti-acetaminophen (Paracetamol) mouse monoclonal antibody (mAb) |
Human acetaminophen (Paracetamol) binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in Paracetamol level test of Pain (hepatotoxicity) and related syndrome evaluation. |
||
Human |
|||||||
Human |
GMP-SMT-PGAg01:BSA-Prostaglandin (PG) ;GMP-SMT-PGAg02: OVA-Prostaglandin (PG) |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-Prostaglandin (PG), Prostaglandin (PG) antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in PG level test of Hormone disorders (acute inflammation) and related syndrome evaluation. |
GMP-SMT-PG-Ab01 Anti-Prostaglandin (PG) mouse monoclonal antibody (mAb) |
Human Prostaglandin (PG) binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in PG level test of Hormone disorders (acute inflammation) and related syndrome evaluation. |
|||
Human |
GMP-SMT-ProgesteroneAg01:BSA-Progesterone ;GMP-SMT-ProgesteroneAg02: OVA-Progesterone |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-Progesterone, Progesterone antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in Progesterone level test of Hormone disorders (fertility) and related syndrome evaluation. |
GMP-SMT-Progesterone-Ab01 Anti-Progesterone mouse monoclonal antibody (mAb) |
Human Progesterone binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in Progesterone level test of Hormone disorders (fertility) and related syndrome evaluation. |
|||
Human |
GMP-SMT-ProlactinAg01:BSA-Prolactin ;GMP-SMT-ProlactinAg02: OVA-Prolactin |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-Prolactin, Prolactin antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in Prolactin level test of Hormone disorders (fertility) and related syndrome evaluation. |
GMP-SMT-Prolactin-Ab01 Anti-Prolactin mouse monoclonal antibody (mAb) |
Human Prolactin binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in Prolactin level test of Hormone disorders (fertility) and related syndrome evaluation. |
|||
Human |
aldosterone (RAA/Aldo) |
GMP-SMT-RAA-AldoAg01:BSA-aldosterone (RAA/Aldo) ;GMP-SMT-RAA-AldoAg02:OVA-aldosterone (RAA/Aldo) |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-aldosterone (RAA/Aldo), aldosterone (RAA/Aldo) antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in RAA/Aldo level test of Cardiovascular disease (Hypertension, blood pressure, hyperaldosteronism, renal artery stenosis (narrowing of
the renal artery), congestive heart failure, cirrhosis, some kidney diseases
(e.g., nephrotic syndrome).) and related syndrome evaluation. |
GMP-SMT-RAA-Aldo-Ab01 Anti-aldosterone (RAA/Aldo) mouse monoclonal antibody (mAb) |
Human aldosterone (RAA/Aldo) binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in RAA/Aldo level test of Cardiovascular disease (Hypertension, blood pressure, hyperaldosteronism, renal artery stenosis (narrowing of
the renal artery), congestive heart failure, cirrhosis, some kidney diseases
(e.g., nephrotic syndrome).) and related syndrome evaluation. |
||
Human |
|||||||
Human |
GMP-SMT-RBP4Ag01:BSA-Retinol binding protein 4 (RBP4) ;GMP-SMT-RBP4Ag02: OVA-Retinol binding protein 4 (RBP4) |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-Retinol binding protein 4 (RBP4), Retinol binding protein 4 (RBP4) antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in RBP4 level test of Metabolic diseases (obesity, type 2 diabetes, metabolic syndrome,
and cardiovascular diseases) and related syndrome evaluation. |
GMP-SMT-RBP4-Ab01 Anti-Retinol binding protein 4 (RBP4) mouse monoclonal antibody (mAb) |
Human Retinol binding protein 4 (RBP4) binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in RBP4 level test of Metabolic diseases (obesity, type 2 diabetes, metabolic syndrome,
and cardiovascular diseases) and related syndrome evaluation. |
|||
Human |
Reverse triiodothyronine (rT3) |
GMP-SMT-rT3Ag01:BSA-Reverse triiodothyronine (rT3) ;GMP-SMT-rT3Ag02: OVA-Reverse triiodothyronine (rT3) |
Competitive immunoassay-validated hapten-carrier conjugates BSA-& OVA-Reverse triiodothyronine (rT3), Reverse triiodothyronine (rT3) antibodies binding, Immunogen in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays as control material in rT3 level test of Thyroid diseases (stress?) and related syndrome evaluation. |
GMP-SMT-rT3-Ab01 Anti-Reverse triiodothyronine (rT3) mouse monoclonal antibody (mAb) |
Human Reverse triiodothyronine (rT3) binding, in Competitive immunoassay validation, Elisa, lateral-flow tests, and other immunoassays in rT3 level test of Thyroid diseases (stress) and related syndrome evaluation. |
||
Human |
Antibiotics
Diagnostic antibodies and antigens for the detection of maximum residue limit(MRL) of antibiotics
Antibiotics are used for veterinary purposes almost as soon as they had been developed for human medicine. They are supplemented in the animal feed for growth promotion and treatment of infections, which are important during the development of intensive methods of animal husbandry, as a result there are major hazards that could be posed by antibiotic residues in foods like raw milk, poultry, cattle, p0rcine, fish and so on. The possible presence of antimicrobial residues (AR) in farm foods poses a risk for consumers such as allergic reactions, toxicity, carcinogenic effects, selection of resistant bacteria, disruption of human normal flora, provoke immunological response and inhibition of the starter culture.
The risk of residue from these foods are higher in developing countries compared to developed one. This might be related with lack of facilities for detection and regulatory bodies that control the drug residues level in foods in the form of maximum residue limits (MRLs). Therefore, to prevent/minimize the risk of antimicrobial residue in milk, different methods of detection of residue to the standard limits level in all food items is possible by chemical methods, microbiological methods and immunological assays. However, Genemedi developed the antigen and antibody to detect the MRL of various antibiotics in the food such as raw milk, poultry, cattle, p0rcine, fish and so on. Detecting the MRL of different antibiotics from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Introdution-Food safety and small molecule detection
Food can transmit disease from person to person by serving as a growth medium for food contaminations that can cause food poisoning. Food safety refers to non-toxic, harmless of food, in line with the nutritional requirements, and does not cause any acute, subacute or chronic hazards on human health. Currently, food safety is a growing concern all over the world especially in developing areas. Ensuring the safety of food has become a significant challenge due to globalization of the food supply and the demand for minimally processed food products. Some potential sources for chemical and biological contamination of foods include: the use of pesticides, fertilizers, and antibiotics around agricultural food products; accidental introduction of additives to industrially processed foods at unsafe levels, or intentional adulteration with low quality or unsafe ingredients for economic purposes; cross-contamination with allergens or other substances that can be dangerous to sensitive individuals (e.g., wheat gluten); microbial growth deriving from unsanitary agricultural or processing conditions; and spoilage during transport and storage of foods due to packaging defects or incorrect handling by consumers. To manage food safety risks, there is a continued need for rapid, sensitive, inexpensive, and reliable techniques to detect the presence of chemical contaminants and microbes in complex media.
Food Safety Solutions
Food testing and analyzing plays an important role in quality control of food production.
1. Culture-based traditional techniques
Traditional culture methods use selective liquid or solid culture media to grow, isolate, and enumerate target microorganism and simultaneously prevent the growth of other microorganisms present in the food (Jasson et al., 2010). These methods for the identification of foodborne pathogens involve pre-enrichment growth, selective enrichment culture, and selective plating followed by biochemical identification and serological confirmation of results. These methods are relatively inexpensive, sensitive, and still regarded as gold standards, the main drawback of these methods is their long analysis time and labor intensiveness. The whole procedure takes typically between 7 and 10 days (Vunrcrzant and Pllustoesser, 1987; Biswas, 2005).
2. Mircoscopic and optical characteristics based methods
Various methods based on microscopic and optical characteristics of the appropriately stained microbial cells have been developed for assuring microbial safety of foods and food products. These methods include Direct Epifluorescent Filter Technique (DEFT), Flow Cytometry, and Solid Phase Cytometry. DEFT is a rapid method for enumerating microbial foodborne pathogens and is used widely in the dairy industry for raw foods (Hermida et al., 2000), milk and milk products, beverages, foods, etc. Flow cytometry is applied for the enumeration of viable bacteria in a sample, and uses flourescent dyes for the analysis of viability, metabolic state, and antigenic markers of bacteria. Solid-phase cytometry (SPC) combines the principles of epifluorescence microscopy and flow cytometry.
3. Immunological methods
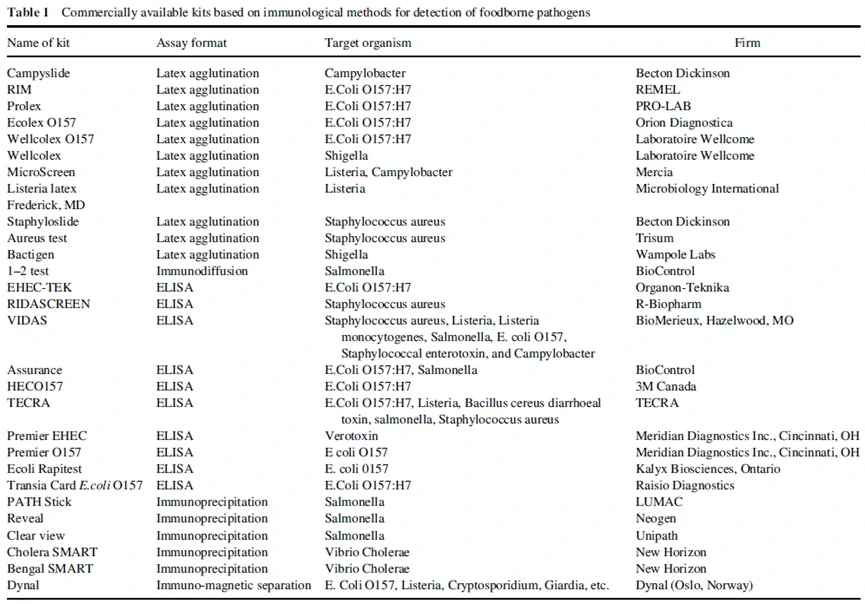
All immunological methods for the detection of foodborne pathogens are based on antigen–antibody reactions. The body produces specific antibodies in response to invading pathogen. These reactions are versatile and specific but the success of an immunoassay depends on the specificity of antibody. Various kits have been developed based on immunological methods for the detection of foodborne pathogens, and are available commercially and have been responsible for revolutionizing the field of food testing (Table 1).
4. Nucleic acid-based methods
Spurred by technological developments and commercial profit motives, nucleic acid-based assays have become widely available as powerful tools to assist in the diagnosis and monitoring of foodborne pathogens. These methods are based on the detection of specific gene sequences (signature sequences) in the genotype of target organism. The sequences may be selected in such a way that they can detect a particular group, genus, species, or even the strain of microorganism. There are many DNA-based assay formats, but probes and nucleic acid amplification techniques are the most popular ones and have been developed commercially for detecting foodborne pathogens.
5. Mass spectrometry
For years, mass spectrometry has been considered the most suitable analytical technique for the detection of multiple compounds in food, feed and water. Coupled to liquid chromatography (LC), high-performance LC and ultra-high performance LC (HPLC, UHPLC) or gas chromatographic (GC) separation with an ionization source such as electrospray (ESI), a large number of mass spectrometry-based methods were developed to comply with updated regulations. To effectively apply this approach, the structure of the compound must be characterized before its detection. Methods development can be time-consuming, and standards must be acquired to optimize compound-specific instrumental conditions, including transition selections, ion-source voltages, and collision energies. MRM methods are, therefore, unable to screen for unknown compounds
Small molecule detection-Hapten based competitive immunoassay
Small molecules such as pesticides, drugs, etc. are usually nonimmunogenic and hence do not elicit an immune response unless coupled with some macromolecules such as proteins. It is, therefore, required to modify these small substances (hapten) for coupling with macromolecules (carrier) so as to make a stable carrierhapten complex. Synthesis of hapten for linking with carrier proteins is the most important aspect of specific antibody generation against small molecules for immunoassay applications.




