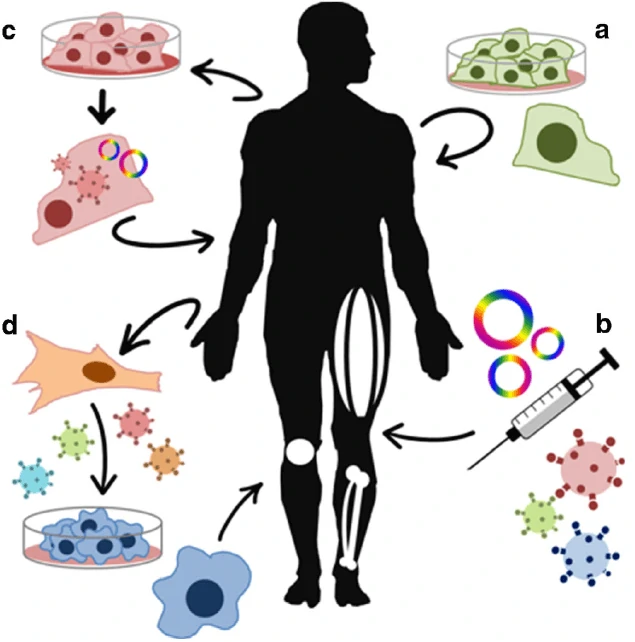
Genetic re-engineering cell therapy in vivo
Cell therapy is a kind of medicine aiming to cure disease or alleviate disease symptoms via direct infusion or transplantation of cells, which can be autologous or allogeneic. With several decades’ development and optimization, immuno-oncology cells (such as T cells, nature killer cells, etc.), stem cells (embryonic stem cells, induced pluripotent stem cells, progenitor cells, etc.) or other genetic re-engineered cells have been widely applied for cell therapy. Numerous cell types have been translated into clinical trials and promising cell therapy outcomes have been achieved from Phase I, Phase II and Phase III trials for a great number of diseases.
Besides the immune-oncology therapy and stem cell derived therapy, gene replacement therapy via lentivirus, adenovirus and adenovirus associated virus (AAV) as well as genetic engineered strategies via CRISPR, ZLN, TALEN have been widely applied in the field of cell therapy [149,150]. For example, PCSK9 gene can be disrupted to prevent cardiovascular disease [151], and PRKAG2 allele (with H530R mutation) can be disrupted to restore the heart morphology and function in Wolff-Parkinson-White syndrome with the wild-type PRKAG2 allele left [152], while pathogenic mutation in the MYBPC3 gene can be corrected in human embryo for the prevention of hypertrophic cardiomyopathy [153]. Besides cardiovascular disease, the mutation in hemoglobin β (HBB) gene was also be seamlessly corrected for the therapy of β-thalassemia [154], and the point mutation in exon 14 of the JAK3 gene in severe combined immunodeficiency (SCID) was corrected to restore the development and maturation ability of T cells [155]. On the basis of the impressive and encouraging outcomes of genetic re-engineering cell therapy, a great number of clinical trials have been carried out to translate the basic scientific achievements into medicines for disease therapy.
Beta-thalassemia and re-engineering cell therapy
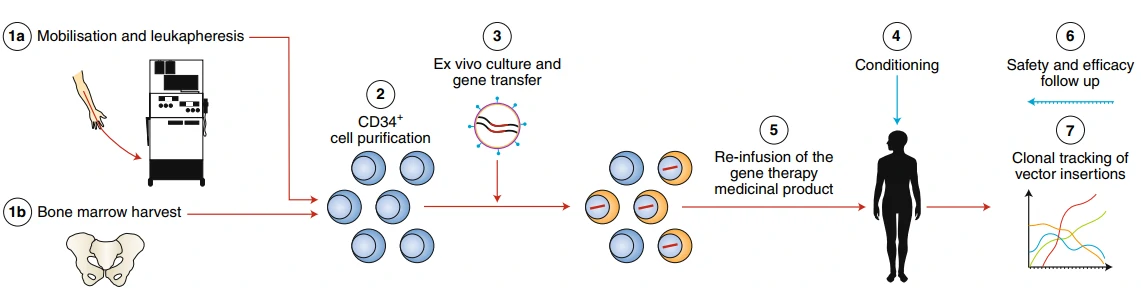
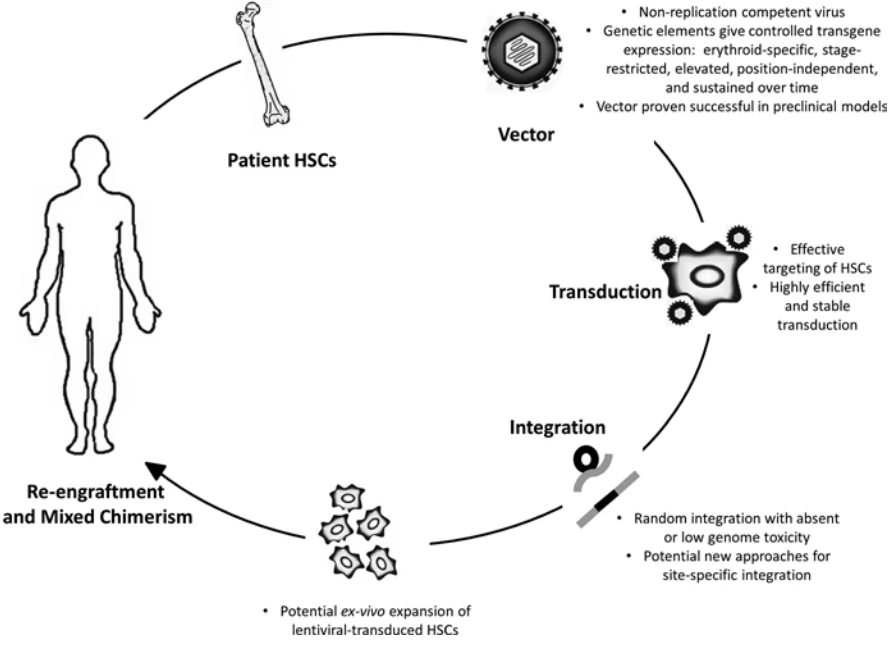
Beta-thalassemia (β-thalassemia), also known as Cooley’s anemia, is a hereditary monogenic hematological disease caused by mutations in the human hemoglobin b (HBB) gene and thus lack of the β-globin chain synthesis [157]. There are two traditional therapeutic methods for the treatment of β-thalassemia: 1) frequent iron chelation therapy, which is easy to cause iron overload and cannot sustain throughout a lifetime [158]; 2) allogeneic hematopoietic stem cell (HSC) transplantation, which is difficult to find a fully-matched donor [159]. Re-engineering cell therapy is a novel therapy without the above drawbacks that utilizes gene editing technology, such as CRISPR, to correct the mutated HBB gene or supplement a functional copy of HBB gene thus restore the synthesis ability of β-globin and cure β-thalassemia (Fig. 12) [160]. Gene therapy by autologous CD34+ cells transduced with the lentiviral BB305 vector, i.e. LentiGlobin, revealed increased hemoglobin levels in patients with no serious adverse effects or no need for long-term red-cell transfusions [161]. To date, LentiGlobin in bluebird bio has been approved to follow the fastest assessment of an advanced therapy medicinal product (ATMP) as part of the European Medicines Agency’s Priority Medicines (PRIME) program.
GSK's Strimvelis for ADA-SCID treatment
Adenosine Deaminase (ADA)-Deficient Severe Combined Immune Deficiency (SCID) is a rare, autosomal recessive immunodeficiency, caused by deleterious mutations in ADA gene, for the production of T lymphocytes. There are three therapeutic methods for the treatment of ADA-SCID [162-164]: 1) allogeneic hematopoietic stem cell transplantation, which is difficult to find a fully-matched donor; 2) enzyme replacement therapy (ERT) with polyethylene glycol-conjugated adenosine deaminase, which needs long-term medication as well as high cost (approx. $200,000–400,000 per year) and exhibits great variable availability in different countries [165,166]; 3) autologous HSCT with gene correction of the hematopoietic stem cells, which can avoid the above drawbacks and shows excellent clinical safety and efficacy over the past 15 years [167-170]. In 2016, Strimvelis (also known as GSK2696273, autologous CD34+ cells re-engineered to express ADA) in GlaxoSmithKline was approved by European Commission for the therapy of ADA-SCID.