Vaccines development for COVID-19/SARS-CoV-2
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

5. Vaccines development for COVID-19/SARS-CoV-2
Coronavirus Disease 2019 (COVID-19) is a novel viral pneumonia caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). First discovered in Wuhan, a city in Hubei province of China, COVID-19 has already broken out throughout the world and posed a great threat to the public health, especially in Europe and North America now. Additionally, person-to-person transmission of COVID-19 disease is reported to be extremely rapid [158-160]. To date, more than one million cases were infected with COVID-19 and over 55,000 deaths occurred. Therefore, it is really urgent and noteworthy to develop the vaccines specific to COVID-19/SARS-CoV-2.
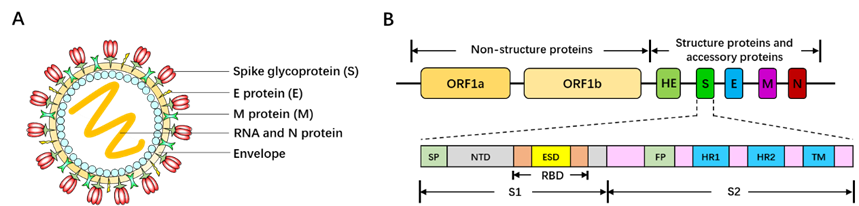
Belonging to the Betacoronavirus genus family, SARS-CoV-2 is 60~200nm in diameter and encapsidates a large positive-sense, single-stranded RNA virus (26-32kb) with many spikes on the virus capsid (Fig. 17A). The RNA genome of SARS-CoV-2 encodes several accessory proteins and structural proteins, such as nucleocapsid (N) protein, envelope (E) protein, membrane (M) protein, and spike (S) protein (Fig. 10B). Although the detailed mechanism of SARS-CoV-2 infection has not been clearly illuminated, several studies demonstrated that SARS-CoV-2 enters human cells via utilizing spike (S) protein to bind to the angiotensin converting enzyme (ACE2) on the surface of target cell [161, 162].
Since the genome sequences of SARS-CoV were discovered and reported (https://www.gisaid.org/CoV2020/), a large number of pharmaceutical enterprises and research organizations are sparing all efforts to the vaccine development. Different companies utilize different targets and antigen epitopes. Some of the advances are listed in the following Table 10 (from WHO), and most of them focus on viral vector-based vaccines (replicating or non-replicating viral vector-based vaccines), recombinant protein (Spike), and nucleic acid-based vaccines. To date, two COVID-19 vaccines have entered Phase I clinical testing to assess the safety and potency of vaccines. One is mRNA-1273, was developed by Moderna Therapeutics, encoding a prefusion-stabilized form of Spike (S) protein [163] (https://www.nature.com/articles/d41587-020-00005-z). Another vaccine is recombinant protein of SARS-CoV-2 antigen, developed by Chinese Academy of Military Sciences, Institute of Military Medicine. It was predicted that these vaccines can be applied in clinics in a large scale as early as 2021 if they can successfully pass the clinical testing. Although there is a long way for theses vaccines to be applied for prevention and therapy of COVID-19, they indeed bring great hope and light to people all over the world.
|
|
|
|
|
|
|
| |||
|
|
|
|
| ||||||
| Sinovac | Inactivated | Inactivated | 2 | 0,14 days | IM |
NCT04383574 NCT04352608 Study Report NCT04551547 |
NCT04456595 669/UN6.KEP/EC/2020 NCT04582344 NCT04617483 |
||
| Wuhan Institute of Biological Products/Sinopharm | Inactivated | Inactivated | 2 | 0,21 days | IM |
ChiCTR2000031809 Interim Report |
ChiCTR2000034780 ChiCTR2000039000 NCT04612972 |
||
| Beijing Institute of Biological Products/Sinopharm | Inactivated | Inactivated | 2 | 0,21 days | IM |
ChiCTR2000032459 Study Report |
ChiCTR2000034780 NCT04560881 |
||
| Bharat Biotech | Inactivated | Whole-Virion Inactivated | 2 | 0, 28 days | IM |
CTRI/2020/07/026300 CTRI/2020/09/027674 |
CTRI/2020/11/028976 NCT04641481 |
||
| University of Oxford/AstraZeneca | Non-Replicating Viral Vector | ChAdOx1-S | 2 | 0,28 days | IM |
PACTR202006922165132 2020-001072-15 NCT04568031 Interim Report |
2020-001228-32 Study Report |
ISRCTN89951424 NCT04516746 NCT04540393 CTRI/2020/08/027170 |
|
| CanSino Biological Inc./Beijing Institute of Biotechnology | Non-Replicating Viral Vector | Adenovirus Type 5 Vector | 1 | IM | ChiCTR2000030906 NCT04568811 Study Report |
ChiCTR2000031781 NCT04566770 Study Report |
NCT04526990 NCT04540419 |
||
| Gamaleya Research Institute | Non-Replicating Viral Vector | Adeno-based (rAd26-S+rAd5-S) | 2 | 0,21 days | IM | NCT04436471 NCT04437875 Study Report |
NCT04587219 NCT04640233 |
NCT04530396 NCT04564716 NCT04642339 |
|
| Janssen Pharmaceutical Companies | Non-Replicating Viral Vector | Adenovirus Type 26 vector | 1 2 | 0 0, 56 days | IM | NCT04436276 NCT04509947 |
NCT04535453 |
NCT04505722 ISRCTN14722499 |
|
| Novavax | Protein Subunit | Full length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | 2 | 0,21 days | IM | NCT04368988 Study Report |
NCT04533399 (phase 2b) |
2020-004123-16 NCT04611802 |
|
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | Protein Subunit | Adjuvanted recombinant protein (RBD-Dimer) expressed in CHO cells | 3 | 0, 28, 56 days | IM | NCT04445194 NCT04636333 |
NCT04550351 |
NCT04466085 |
ChiCTR2000040153 |
| Moderna/NIAID | RNA | LNP-encapsulated mRNA | 2 | 0,28 days | IM | NCT04283461 Interim Report Final Report |
NCT04405076 |
NCT04470427 |
|
| BioNTech/Fosun Pharma/Pfizer | RNA | 3 LNP-mRNAs | 2 | 0,21 days | IM | NCT04368728 Study Report |
2020-001038-36 ChiCTR2000034825 NCT04537949 NCT04588480 Study Report1 Study Report2 |
NCT04368728 |
|
| Medicago Inc. | VLP | Plant-derived VLP adjuvanted with AS03. | 2 | 0, 21 days | IM | NCT04450004 |
NCT04636697 |
||
| Inovio Pharmaceuticals/ International Vaccine Institute | DNA | DNA plasmid vaccine with electroporation | 2 | 0, 28 days | ID | NCT04447781 NCT04336410 |
NCT04642638 ChiCTR2000040146 |
||
| Beijing Wantai Biological Pharmacy/ Xiamen University | Replicating Viral Vector | Intranasal flu-based-RBD | 1 | IN | |||||
| West China Hospital, Sichuan University | Protein Subunit | RBD (baculovirus production expressed in Sf9 cells) | 2 or 3 | 0, 28 days and 0,14, 28 days | IM | ChiCTR2000039994 |
|||
| Curevac | RNA | mRNA | 2 | 0, 28 days | IM | NCT04449276 |
NCT04515147 |
||
| Institute of Medical Biology, Chinese Academy of Medical Sciences | Inactivated | Inactivated | 2 | 0, 28 days | IM | NCT04412538 |
NCT04470609 |
||
| Research Institute for Biological Safety Problems, Rep of Kazakhstan | Inactivated | Inactivated | 2 | 0, 21 days | IM | NCT04530357 |
|||
| Shenzhen Kangtai Biological Products Co., Ltd. | Inactivated | Inactivated | 2 | IM | ChiCTR2000038804 |
ChiCTR2000039462 |
|||
| Osaka University/ AnGes/ Takara Bio | DNA | DNA plasmid vaccine + Adjuvant | 2 | 0, 14 days | IM | NCT04463472 NCT04527081 |
|||
| Cadila Healthcare Limited | DNA | DNA plasmid vaccine | 3 | 0, 28, 56 days | ID | CTRI/2020/07/026352 |
|||
| Genexine Consortium | DNA | DNA Vaccine (GX-19) | 2 | 0, 28 days | IM | NCT04445389 |
|||
| Kentucky Bioprocessing, Inc | Protein Subunit | RBD-based | 2 | 0, 21 days | IM | NCT04473690 |
|||
| Sanofi Pasteur/GSK | Protein Subunit | S protein (baculovirus production) | 2 | 0, 21 days | IM | NCT04537208 |
|||
| Biological E Ltd | Protein Subunit | Adjuvanted protein subunit (RBD) | 2 | 0, 28 days | IM | CTRI/2020/11/029032 |
|||
| Israel Institute for Biological Research | Replicating Viral Vector | VSV-S | 1 | IM | NCT04608305 |
||||
| Arcturus/Duke-NUS | RNA | mRNA | IM | NCT04480957 |
|||||
| SpyBiotech/Serum Institute of India | VLP | RBD-HBsAg VLPs | 2 | 0, 28 days | IM | ACTRN12620000817943 |
|||
| Symvivo | DNA | bacTRL-Spike | 1 | Oral | NCT04334980 |
||||
| Providence Health & Services | DNA | electroporated S protein plasmid DNA vaccine with or without the combination of electroporated IL- 12p70 plasmid | 2 | 0, 28 days | ID | NCT04627675 |
|||
| Codagenix/Serum Institute of India | Live Attenuated Virus | Codon deoptimized live attenuated vaccines | 1 or 2 | 0 or 0,28 days | IN | NCT04619628 |
|||
| ImmunityBio, Inc. & NantKwest Inc. | Non-Replicating Viral Vector | hAd5 S+N 2nd Generation Human Adenovirus Type 5 Vector (hAd5) Spike (S) + Nucleocapsid (N) | 2 | 0, 21 days | SC | NCT04591717 |
|||
| ReiThera/LEUKOCARE/Univercells | Non-Replicating Viral Vector | Replication defective Simian Adenovirus (GRAd) encoding S | 1 | IM | NCT04528641 | ||||
| CanSino Biological Inc/Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | Non-Replicating Viral Vector | Ad5-nCoV | 2 | 0, 28 days | IM/mucosal | NCT04552366 | |||
| Vaxart | Non-Replicating Viral Vector | Ad5 adjuvanted Oral Vaccine platform | 2 | 0, 28 days | Oral | NCT04563702 | |||
| Ludwig-Maximilians – University of Munich | Non-Replicating Viral Vector | MVA-SARS-2-S | 2 | 0, 28 days | IM | NCT04569383 | |||
| City of Hope, USA | Replicating Viral Vector | SARS-CoV-2 S and NP genes inserted into a sMVA vector | 2 | 0, 28 days | IM | NCT04639466 | |||
| Clover Biopharmaceuticals Inc./GSK/Dynavax | Protein Subunit | Native like Trimeric subunit Spike Protein vaccine | 2 | 0, 21 days | IM | NCT04405908 | |||
| Vaxine Pty Ltd/Medytox | Protein Subunit | Recombinant spike protein with Advax™ adjuvant | 1 | IM | NCT04453852 | ||||
| University of Queensland/CSL/Seqirus | Protein Subunit | Molecular clamp stabilized Spike protein with MF59 adjuvant | 2 | 0, 28 days | IM | ACTRN12620000674932p ISRCTN51232965 |
|||
| Medigen Vaccine Biologics Corporation/NIAID/Dynavax | Protein Subunit | S-2P protein + CpG 1018 | 2 | 0, 28 days | IM | NCT04487210 | |||
| Instituto Finlay de Vacunas, Cuba | Protein Subunit | rRBD produced in CHO-cell chemically conjugate to tetanus toxoid | 2 | 0, 28 days | IM | IFV/COR/06 | |||
| Instituto Finlay de Vacunas, Cuba | Protein Subunit | RBD + Adjuvant | 2 | 0, 28 days | IM | IFV/COR/04 IFV/COR/05 |
|||
| FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | Protein Subunit | Peptide | 2 | 0, 21 days | IM | NCT04527575 | |||
| University Hospital Tuebingen | Protein Subunit | SARS-CoV-2 HLA-DR peptides | 1 | SC | NCT04546841 | ||||
| COVAXX / United Biomedical Inc. Asia | Protein Subunit | Multitope peptide-based S1-RBD- protein vaccine | 2 | 0, 28 days | IM | NCT04545749 | |||
| Chinese Academy of Military Sciences | Protein Subunit | Subunit expressed in CHO cells | 2 or 3 | 0, 14 days or 0,14, 28 days | IM | ||||
| Merck Sharp & Dohme/IAVI | Replicating Viral Vector | Replication-competent VSV delivering the SARS-CoV-2 Spike | 1 | IM | NCT04569786 | ||||
| Institute Pasteur/Themis/Univ. of Pittsburg CVR/Merck Sharp & Dohme | Replicating Viral Vector | Measles-vector based | 1 or 2 | 0, 28 days | IM | NCT04497298 | |||
| Imperial College London | RNA | LNP-nCoVsaRNA | 2 | IM | ISRCTN17072692 | ||||
| People’s Liberation Army (PLA) Academy of Military Sciences/Walvax Biotech. | RNA | mRNA | 2 | 0, 14 or 0, 28 days | IM | ChiCTR2000034112 ChiCTR2000039212 |
|||
| Platform | Type of candidate vaccine | Developer | Coronavirus target | Current stage of clinical evaluation/regulatory status- Coronavirus candidate | Same platform for non-Coronavirus candidates | |||||||||||
| DNA | DNA plasmids containing S-gene | Biosun Pharmed | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA plasmid vaccine | Globe Biotech Limited, Bangladesh | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | Plasmid DNA, nanostructured RBD | National institute of Chemistry, Slovenia | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA, engineered vaccine inserts compatible with multiple delivery systems | DIOSynVax Ltd / University of Cambridge | SARS-CoV-2 and Sarbeco-CoV | Pre-Clinical | ||||||||||||
| DNA | DNA vaccine | Ege University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA plasmid vaccine RBD&N | Scancell/University of Nottingham/ Nottingham Trent University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA plasmid vaccine S,S1,S2,RBD &N | National Research Centre, Egypt | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA with electroporation | Karolinska Institute / Cobra Biologics (OPENCORONA Project) | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA with electroporation | Chula Vaccine Research Center | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA | Takis/Applied DNA Sciences/Evvivax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | Plasmid DNA, Needle-Free Delivery | Immunomic Therapeutics, Inc./EpiVax, Inc./PharmaJet | SARS-CoV2 | Pre-Clinical | SARS | |||||||||||
| DNA | DNA vaccine | BioNet Asia | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | msDNA vaccine | Mediphage Bioceuticals/University of Waterloo | SARS-CoV2 | Pre-Clinical | ||||||||||||
| DNA | DNA vaccine | Entos Pharmaceuticals | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated + Alum | Shifa Pharmed | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated | Milad Pharmaceutics Co. | SARS-CoV2 | Pre-Clinical | MMR, IPV | |||||||||||
| Inactivated | Inactivated | Zista Kian Azma Co. | SARS-CoV2 | Pre-Clinical | MMR, IPV | |||||||||||
| Inactivated | Inactivated | Kocak Farma Ilac ve Kimya San. A.S. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Egg-based, inactivated, whole chimeric Newcastle Disease Virus (NDV) expressing membrane-anchored pre-fusion-stabilized trimeric SARS-CoV-2 S protein (Hexapro) + CpG 1018 | Institute of Vaccines and Medical Biologicals (IVAC; Vietnam) / Dynavax / PATH | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Egg-based, inactivated, whole chimeric Newcastle Disease Virus (NDV) expressing membrane-anchored pre-fusion-stabilized trimeric SARS-CoV-2 S protein (Hexapro) + CpG 1018 | Government Pharmaceutical Organization (GPO; Thailand) / Dynavax / PATH | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Egg-based, inactivated, whole chimeric Newcastle Disease Virus (NDV) expressing membrane-anchored pre-fusion-stabilized trimeric SARS-CoV-2 S protein (Hexapro) + CpG 1018 | Institute Butantan (Brazil) / Dynavax / PATH | SARS-CoV-2 | Pre-clinical | ||||||||||||
| Inactivated | Inactivated + alum | KM Biologics | SARS-CoV2 | Pre-Clinical | JE, Zika | |||||||||||
| Inactivated | Inactivated | Selcuk University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated | Erciyes University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated whole virus | National Research Centre, Egypt | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated | SARS-CoV2 | Pre-Clinical | |||||||||||||
| Inactivated | TBD | Osaka University/ BIKEN/ NIBIOHN | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated + CpG 1018 | Sinovac/Dynavax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Inactivated | Inactivated + CpG 1018 | Valneva/Dynavax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Live Attenuated Virus | Codon deoptimized live attenuated vaccines | Mehmet Ali Aydinlar University / Acıbadem Labmed Health Services A.S. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Live Attenuated Virus | Codon deoptimized live attenuated vaccines | Indian Immunologicals Ltd/Griffith University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non Replicating Viral Vector | Ad 5 vector for intranasal administration | University of Helsinki & University of Eastern Finland | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non Replicating Viral Vector | Adenovirus Type 5 Vector | Globe Biotech Limited, Bangladesh | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | Sendai virus vector | ID Pharma | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | Adenovirus-based | Ankara University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | Adeno-associated virus vector (AAVCOVID) | Massachusetts Eye and Ear/Massachusetts General Hospital/AveXis | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | MVA encoded VLP | GeoVax/BravoVax | SARS-CoV2 | Pre-Clinical | LASV, EBOV, MARV, HIV | |||||||||||
| Non-replicating viral vector | MVA-S encoded | DZIF – German Center for Infection Research/IDT Biologika GmbH | SARS-CoV2 | Pre-clinical | Many | |||||||||||
| Non-replicating viral vector | MVA-S | IDIBAPS-Hospital Clinic, Spain | SARS-CoV2 | Pre-clinical | ||||||||||||
| Non-Replicating Viral Vector | Intranasal Ad5 vaccine encoding RBD | Altimmune, Inc. | SARS-CoV2 | IND filed | Influenza (NasoVAX), Anthrax (NasoShield) | |||||||||||
| Non-Replicating Viral Vector | Adeno5-based | Erciyes University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | Ad5 S (GREVAX™ platform) | Greffex | SARS-CoV2 | Pre-Clinical | MERS | |||||||||||
| Non-Replicating Viral Vector | Oral Ad5 S | Stabilitech Biopharma Ltd | SARS-CoV2 | Pre-Clinical | Zika, VZV, HSV-2 and Norovirus | |||||||||||
| Non-Replicating Viral Vector | adenovirus-based + HLA-matched peptides | Valo Therapeutics Ltd | Pan-Corona | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | Vaxart | SARS-CoV2 | Pre-Clinical | InfA, CHIKV, LASV, NORV; EBOV, RVF, HBV, VEE | ||||||||||||
| Non-Replicating Viral Vector | MVA expressing structural proteins | Centro Nacional Biotecnología (CNB-CSIC), Spain | SARS-CoV2 | Pre-Clinical | Multiple candidates | |||||||||||
| Non-Replicating Viral Vector | parainfluenza virus 5 (PIV5)-based vaccine expressing the spike protein | University of Georgia/University of Iowa | SARS-CoV2 | Pre-Clinical | MERS | |||||||||||
| Non-Replicating Viral Vector | Recombinant deactivated rabies virus containing S1 | Bharat Biotech/Thomas Jefferson University | SARS-CoV2 | Pre-Clinical | HeV, NiV, EBOV, LASSA, CCHFV, MERS | |||||||||||
| Non-Replicating Viral Vector | Influenza A H1N1 vector | National Research Centre, Egypt | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Non-Replicating Viral Vector | Newcastle disease virus expressing S | Icahn School of Medicine at Mount Sinai | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant spike with adjuvant | Iran | SARS-CoV2 | Pre-Clinical | Multiple candidates | |||||||||||
| Protein Subunit | Recombinant S protein produced in BEVS | Tampere University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | RBD protein delivered in mannose- conjugated chitosan nanoparticle | Ohio State University / Kazakh National Agrarian University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant spike protein with Essai O/W 1849101 adjuvant | Kazakh National Agrarian University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Peptides | Neo7Logic | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant spike protein with Essai O/W 1849101 adjuvant | Kazakh National Agrarian University, Kazakhstan / National Scientific Center for Especially Dangerous Infections | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant S protein | Max-Planck-Institute of Colloids and Interfaces | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | RBD protein (baculovirus production) + FAR- Squalene adjuvant | Farmacológicos Veterinarios SAC (FARVET SAC) / Universidad Peruana Cayetano Heredia (UPCH) | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Protein Subunit | Research Institute for Biological Safety Problems, Rep of Kazakhstan | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | RBD-protein | Mynvax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant S protein | Izmir Biomedicine and Genome Center | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Peptide + novel adjuvant | Bogazici University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | S subunit intranasal liposomal formulation with GLA/3M052 adjs. | University of Virginia | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | S-Protein (Subunit) + Adjuvant, E coli based Expression | Helix Biogen Consult, Ogbomoso & Trinity Immonoefficient Laboratory, Ogbomoso, Oyo State, Nigeria. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Protein Subunit S,N,M&S1 protein | National Research Centre, Egypt | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Protein Subunit | University of San Martin and CONICET, Argentina | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | RBD protein fused with Fc of IgG + Adj. | Chulalongkorn University/GPO, Thailand | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Capsid-like Particle | AdaptVac (PREVENT-nCoV consortium) | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Drosophila S2 insect cell expression system VLPs | ExpreS2ion | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Peptide antigens formulated in LNP | IMV Inc | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | S protein | WRAIR/USAMRIID | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | S protein +Adjuvant | National Institute of Infectious Disease, Japan/Shionogi/UMN Pharma | SARS-CoV2 | Pre-Clinical | Influenza | |||||||||||
| Protein Subunit | VLP-recombinant protein + Adjuvant | Osaka University/ BIKEN/ National Institutes of Biomedical Innovation, Japan | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | microneedle arrays S1 subunit | Univ. of Pittsburgh | SARS-CoV2 | Pre-Clinical | MERS | |||||||||||
| Protein Subunit | Peptide | Vaxil Bio | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Peptide | Flow Pharma Inc | SARS-CoV2 | Pre-Clinical | Ebola, Marburg, HIV, Zika, Influenza, HPV therapeutic vaccine, BreastCA vaccine | |||||||||||
| Protein Subunit | S protein | AJ Vaccines | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Ii-Key peptide | Generex/EpiVax | SARS-CoV2 | Pre-Clinical | Influenza, HIV, SARS-CoV | |||||||||||
| Protein Subunit | S protein | EpiVax/Univ. of Georgia | SARS-CoV2 | Pre-Clinical | H7N9 | |||||||||||
| Protein Subunit | Protein Subunit EPV-CoV-19 | EpiVax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | gp-96 backbone | Heat Biologics/Univ. Of Miami | SARS-CoV2 | Pre-Clinical | NSCLC, HIV, malaria, Zika | |||||||||||
| Protein Subunit | Subunit vaccine | FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | S1 or RBD protein | Baylor College of Medicine | SARS-CoV2 | Pre-Clinical | SARS | |||||||||||
| Protein Subunit | Subunit protein, plant produced | iBio/CC-Pharming | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant protein, nanoparticles (based on S-protein and other epitopes) | Saint-Petersburg scientific research institute of vaccines and serums | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | COVID-19 XWG-03 truncated S (spike) proteins | Innovax/Xiamen Univ./GSK | SARS-CoV2 | Pre-Clinical | HPV | |||||||||||
| Protein Subunit | Adjuvanted microsphere peptide | VIDO-InterVac, University of Saskatchewan | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Synthetic Long Peptide Vaccine candidate for S and M proteins | OncoGen | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Oral E. coli-based protein expression system of S and N proteins | MIGAL Galilee Research Institute | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Nanoparticle vaccine | LakePharma, Inc. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Plant-based subunit (RBD-Fc + Adjuvant) | Baiya Phytopharm/ Chula Vaccine Research Center | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | OMV-based vaccine | Quadram Institute Biosciences | SARS-CoV2 | Pre-Clinical | Flu A, plague | |||||||||||
| Protein Subunit | OMV-based vaccine | BiOMViS Srl/Univ. of Trento | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein subunit | structurally modified spherical particles of the tobacco mosaic virus (TMV) | Lomonosov Moscow State University | SARS-CoV2 | Pre-Clinical | rubella, rotavirus | |||||||||||
| Protein Subunit | Spike-based | University of Alberta | SARS-CoV2 | Pre-Clinical | Hepatitis C | |||||||||||
| Protein Subunit | Recombinant S1-Fc fusion protein | AnyGo Technology | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant protein | Yisheng Biopharma | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Recombinant S protein in IC-BEVS | Vabiotech | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Orally delivered, heat stable subunit | Applied Biotechnology Institute, Inc. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Peptides derived from Spike protein | Axon Neuroscience SE | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Protein Subunit | MOGAM Institute for Biomedical Research, GC Pharma | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | RBD-based | Neovii/Tel Aviv University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Outer Membrane Vesicle (OMV)-subunit | Intravacc/Epivax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Outer Membrane Vesicle(OMV)-peptide | Intravacc/Epivax | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Spike-based (epitope screening) | ImmunoPrecise/LiteVax BV | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Protein Subunit | Spiked-based | Nanografi Nano Technology, Middle East Technical University, Ankara University, | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Bacteria Vector | Oral Salmonella enteritidis (3934Vac) based protein expression system of RBD | Farmacológicos Veterinarios SAC (FARVET SAC) / Universidad Peruana Cayetano Heredia (UPCH) | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | Intranasal Newcastle disease virus vector (rNDV-FARVET) expressing RBD | Farmacológicos Veterinarios SAC (FARVET SAC) / Universidad Peruana Cayetano Heredia (UPCH) | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | YF17D Vector | KU Leuven | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | Measles Vector | Cadila Healthcare Limited | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | Measles Vector | FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | Measles Virus (S, N targets) | DZIF – German Center for Infection Research/CanVirex AG | SARS-CoV2 | Pre-clinical | Zika, H7N9, CHIKV | |||||||||||
| Replicating Viral Vector | Horsepox vector expressing S protein | Tonix Pharma/Southern Research | SARS-CoV2 | Pre-Clinical | Smallpox, monkeypox | |||||||||||
| Replicating Viral Vector | Live viral vectored vaccine based on attenuated influenza virus backbone (intranasal) | BiOCAD and IEM | SARS-CoV2 | Pre-Clinical | Influenza | |||||||||||
| Replicating Viral Vector | Recombinant vaccine based on Influenza A virus, for the prevention of COVID-19 (intranasal) | FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | SARS-CoV2 | Pre-Clinical | Influenza | |||||||||||
| Replicating Viral Vector | Attenuated Influenza expressing an antigenic portion of the Spike protein | Fundação Oswaldo Cruz and Instituto Buntantan | SARS-CoV2 | Pre-Clinical | Influenza | |||||||||||
| Replicating Viral Vector | Influenza vector expressing RBD | University of Hong Kong | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | Replicating VSV vector-based DC-targeting | University of Manitoba | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | VSV-S | University of Western Ontario | SARS-CoV2 | Pre-Clinical | HIV, MERS | |||||||||||
| Replicating Viral Vector | VSV-S | Aurobindo | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | VSV vector | FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | M2-deficient single replication (M2SR) influenza vector | UW–Madison/FluGen/Bharat Biotech | SARS-CoV2 | Pre-Clinical | influenza | |||||||||||
| Replicating Viral Vector | Newcastle disease virus vector (NDV-SARS- CoV-2/Spike) | Intravacc/ Wageningen Bioveterinary Research/Utrecht Univ. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| Replicating Viral Vector | Avian paramyxovirus vector (APMV) | The Lancaster University, UK | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | Providence Therapeutics | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | Cell Tech Pharmed | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | ReNAP Co. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | D614G variant LNP-encapsulated mRNA | Globe Biotech Ltd | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | saRNA formulated in a NLC | Infectious Disease Research Institute/ Amyris, Inc. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-encapsulated mRNA encoding S | Max-Planck-Institute of Colloids and Interfaces | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | Self-amplifying RNA | Gennova | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | Selcuk University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-mRNA | Translate Bio/Sanofi Pasteur | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-mRNA | CanSino Biologics/Precision NanoSystems | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-encapsulated mRNA cocktail encoding VLP | Fudan University/ Shanghai JiaoTong University/RNACure Biopharma | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-encapsulated mRNA encoding RBD | Fudan University/ Shanghai JiaoTong University/RNACure Biopharma | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | Replicating Defective SARS-CoV-2 derived RNAs | Centro Nacional Biotecnología (CNB-CSIC), Spain | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-encapsulated mRNA | University of Tokyo/ Daiichi-Sankyo | SARS-CoV2 | Pre-Clinical | MERS | |||||||||||
| RNA | Liposome-encapsulated mRNA | BIOCAD | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | Several mRNA candidates | RNAimmune, Inc. | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | China CDC/Tongji University/Stermina | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | LNP-mRNA | Chula Vaccine Research Center/University of Pennsylvania | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA in an intranasal delivery system | eTheRNA | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | Greenlight Biosciences | SARS-CoV2 | Pre-Clinical | ||||||||||||
| RNA | mRNA | IDIBAPS-Hospital Clinic, Spain | SARS-CoV2 | Pre-Clinical | ||||||||||||
| T-cell based | CD8 T cell peptide targeting (S, M, N) and (NSPs) SARS-CoV-2 proteins | OSE immunotherapeutics | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | Plant derived VLP | Shiraz University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | VLPs produced in BEVS | Tampere University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | VLP | Max Planck Institute for Dynamics of Complex Technical Systems | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | Virus-like particle-based Dendritic Cell(DC)- targeting vaccine | University of Manitoba | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | VLP | Bezmialem Vakif University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | VLP | Middle East Technical University | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | Enveloped Virus-Like Particle (eVLP) | VBI Vaccines Inc. | SARS-CoV-2, SARS-CoV, MERS-CoV | Pre-Clinical | CMV, GBM, Zika | |||||||||||
| VLP | S protein integrated in HIV VLPs | IrsiCaixa AIDS Research/IRTA-CReSA/Barcelona Supercomputing Centre/Grifols | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | VLP + Adjuvant | Mahidol University/ The Government Pharmaceutical Organization (GPO)/Siriraj Hospital | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | Virus-like particles, lentivirus and baculovirus vehicles | Navarrabiomed, Oncoimmunology group | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | Virus-like particle, based on RBD displayed on virus-like particles | Saiba GmbH | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | ADDomerTM multiepitope display | Imophoron Ltd and Bristol University’s Max Planck Centre | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | Unknown | Doherty Institute | SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | VLP | OSIVAX | SARS-CoV1 SARS-CoV2 | Pre-Clinical | ||||||||||||
| VLP | eVLP | ARTES Biotechnology | SARS-CoV2 | Pre-Clinical | malaria | |||||||||||
| VLP | VLPs peptides/whole virus | Univ. of Sao Paulo | SARS-CoV2 | Pre-Clinical | ||||||||||||
8. References
1.Ura T, Okuda K, Shimada M. Developments in Viral Vector-Based Vaccines. Vaccines (Basel). 2014;2:624-641.
2.Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune Responses to Viral Gene Therapy Vectors. Mol Ther. 2020;28:709-722.
3.Atchison RW, Casto BC, Hammon WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149:754-756.
4.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proceedings of the National Academy of Sciences of the United States of
5.Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. Journal of virology. 2000;74:2777-2785.
6.Ding W, Zhang L, Yan Z, Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene therapy. 2005;12:873-880.
7.Srivastava A. Adeno-associated virus-mediated gene transfer. Journal of cellular biochemistry. 2008;105:17-24.
8.Berns KI. Parvovirus replication. Microbiological reviews. 1990;54:316-329.
9.Pereira DJ, McCarty DM, Muzyczka N. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. Journal of virology. 1997;71:1079-1088.
10.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388-2398.
11.Hosel M, Broxtermann M, Janicki H, Esser K, Arzberger S, Hartmann P, et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55:287-297.
12.Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688-1
13.Shirley JL, Keeler GD, Sherman A, Zolotukhin I, Markusic DM, Hoffman BE, et al. Type I IFN Sensing by cDCs and CD4(+) T Cell Help Are Both Requisite for Cross-Priming of AAV Capsid-Specific CD8(+) T Cells. Mol Ther. 2020;28:758-770.
14.Sudres M, Cire S, Vasseur V, Brault L, Da Rocha S, Boisgerault F, et al. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther. 2012;20:1571-1581.
15.Rabinowitz J, Chan YK, Samulski RJ. Adeno-associated Virus (AAV) versus Immune Response. Viruses. 2019;11.
16.Weitzman MD, Linden RM. Adeno-associated virus biology. Methods in molecular biology. 2011;807:1-23.
17.Vectors used in gene therapy clinical trials. The Journal of Gene Medicine Online Library. [Online] Updated Nov 2017.
18.Kassner U, Hollstein T, Grenkowitz T, Wuhle-Demuth M, Salewsky B, Demuth I, et al. Gene Therapy in Lipoprotein Lipase Deficiency: Case Report on the First Patient Treated with Alipogene Tiparvovec Under Daily Practice Conditions. Hum
19.Passini MA, Bu J, Richards AM, Treleaven CM, Sullivan JA, O’Riordan CR, et al. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Human gene therapy. 2014;25
20.Stieger K, Lorenz B. [Specific gene therapy for hereditary retinal dystrophies – an update]. Klinische Monatsblatter fur Augenheilkunde. 2014;231:210-215.
21.Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO molecular medicine. 2014;6:194-211.
22.Doi K, Takeuchi Y. Gene therapy using retrovirus vectors: vector development and biosafety at clinical trials. Uirusu. 2015;65:27-36.
23.Duncan GA, Kim N, Colon-Cortes Y, Rodriguez J, Mazur M, Birket SE, et al. An Adeno-Associated Viral Vector Capable of Penetrating the Mucus Barrier to Inhaled Gene Therapy. Molecular therapy. Methods & clinical development. 2018;9:29
24.Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Molecular therapy : the journal of the American Society of Gene Therap
25.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. The New England journal of medicine. 2011;365:2357-2365.
26.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reti
27.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. The Lancet. Neurology. 2011;10:309-319.
28.Aalbers CJ, Bevaart L, Loiler S, de Cortie K, Wright JF, Mingozzi F, et al. Preclinical Potency and Biodistribution Studies of an AAV 5 Vector Expressing Human Interferon-beta (ART-I02) for Local Treatment of Patients with Rheumatoid
29.Bevaart L, Aalbers CJ, Vierboom MP, Broekstra N, Kondova I, Breedveld E, et al. Safety, Biodistribution, and Efficacy of an AAV-5 Vector Encoding Human Interferon-Beta (ART-I02) Delivered via Intra-Articular Injection in Rhesus Monke
30.Xin KQ, Urabe M, Yang J, Nomiyama K, Mizukami H, Hamajima K, et al. A novel recombinant adeno-associated virus vaccine induces a long-term humoral immune response to human immunodeficiency virus. Hum Gene Ther. 2001;12:1047-1061.
31.Xin KQ, Ooki T, Mizukami H, Hamajima K, Okudela K, Hashimoto K, et al. Oral administration of recombinant adeno-associated virus elicits human immunodeficiency virus-specific immune responses. Hum Gene Ther. 2002;13:1571-1581.
32.Xin KQ, Mizukami H, Urabe M, Toda Y, Shinoda K, Yoshida A, et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J
33.Lin J, Calcedo R, Vandenberghe LH, Bell P, Somanathan S, Wilson JM. A new genetic vaccine platform based on an adeno-associated virus isolated from a rhesus macaque. J Virol. 2009;83:12738-12750.
34.Nieto K, Stahl-Hennig C, Leuchs B, Muller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum Gene Ther. 2012;23:733-741.
35.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. Journal of virology. 2005;79:9933-9944.
36.Fitzpatrick Z, Leborgne C, Barbon E, Masat E, Ronzitti G, van Wittenberghe L, et al. Influence of Pre-existing Anti-capsid Neutralizing and Binding Antibodies on AAV Vector Transduction. Mol Ther Methods Clin Dev. 2018;9:119-129.
37.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15:445-451.
38.Grimm D, Zolotukhin S. E Pluribus Unum: 50 Years of Research, Millions of Viruses, and One Goal–Tailored Acceleration of AAV Evolution. Mol Ther. 2015;23:1819-1831.
39.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nature biotechnology. 2006;24:198-204.
40.Khabou H, Desrosiers M, Winckler C, Fouquet S, Auregan G, Bemelmans AP, et al. Insight into the mechanisms of enhanced retinal transduction by the engineered AAV2 capsid variant -7m8. Biotechnology and bioengineering. 2016;113:2712-2
41.Kienle E, Senis E, Borner K, Niopek D, Wiedtke E, Grosse S, et al. Engineering and evolution of synthetic adeno-associated virus (AAV) gene therapy vectors via DNA family shuffling. Journal of visualized experiments : JoVE. 2012.
42.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proceedings of the Society for Experimental Biology and Medicine. So
43.Wong CM, McFall ER, Burns JK, Parks RJ. The role of chromatin in adenoviral vector function. Viruses. 2013;5:1500-1515.
44.Ranki T, Hemminki A. Serotype chimeric human adenoviruses for cancer gene therapy. Viruses. 2010;2:2196-2212.
45.Ison MG, Hayden RT. Adenovirus. Microbiology spectrum. 2016;4.
46.Vorburger SA, Hunt KK. Adenoviral gene therapy. The oncologist. 2002;7:46-59.
47.Seiler MP, Cerullo V, Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther. 2007;7:297-305.
48.Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood. 2007;109:2832-2839.
49.Atasheva S, Shayakhmetov DM. Adenovirus sensing by the immune system. Curr Opin Virol. 2016;21:109-113.
50.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, et al. Coagulation factor X activates innate immunity to human species C adenovirus. Science. 2012;338:795-798.
51.Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1256070.
52.Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554-2561.
53.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478-7491.
54.Allen RJ, Byrnes AP. Interaction of adenovirus with antibodies, complement, and coagulation factors. FEBS Lett. 2019;593:3449-3460.
55.Cotter MJ, Zaiss AK, Muruve DA. Neutrophils interact with adenovirus vectors via Fc receptors and complement receptor 1. J Virol. 2005;79:14622-14631.
56.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol. 2013;14:327-336.
57.Fletcher AJ, James LC. Coordinated Neutralization and Immune Activation by the Cytosolic Antibody Receptor TRIM21. J Virol. 2016;90:4856-4859.
58.Khare R, Hillestad ML, Xu Z, Byrnes AP, Barry MA. Circulating antibodies and macrophages as modulators of adenovirus pharmacology. J Virol. 2013;87:3678-3686.
59.Bottermann M, Foss S, van Tienen LM, Vaysburd M, Cruickshank J, O’Connell K, et al. TRIM21 mediates antibody inhibition of adenovirus-based gene delivery and vaccination. Proc Natl Acad Sci U S A. 2018;115:10440-10445.
60.Di Paolo NC, Baldwin LK, Irons EE, Papayannopoulou T, Tomlinson S, Shayakhmetov DM. IL-1alpha and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PL
61.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110-121.
62.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103-107.
63.Suzuki M, Bertin TK, Rogers GL, Cela RG, Zolotukhin I, Palmer DJ, et al. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol Ther. 2013;21:796-80
64.Anghelina D, Lam E, Falck-Pedersen E. Diminished Innate Antiviral Response to Adenovirus Vectors in cGAS/STING-Deficient Mice Minimally Impacts Adaptive Immunity. J Virol. 2016;90:5915-5927.
65.Wang L, Wen M, Cao X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365.
66.Avgousti DC, Herrmann C, Kulej K, Pancholi NJ, Sekulic N, Petrescu J, et al. A core viral protein binds host nucleosomes to sequester immune danger signals. Nature. 2016;535:173-177.
67.Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, et al. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225-235.
68.Amalfitano A, Parks RJ. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Current gene therapy. 2002;2:111-133.
69.Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther. 2015;15:337-351.
70.Sweeney K, Hallden G. Oncolytic adenovirus-mediated therapy for prostate cancer. Oncolytic virotherapy. 2016;5:45-57.
71.Castro JE, Melo-Cardenas J, Urquiza M, Barajas-Gamboa JS, Pakbaz RS, Kipps TJ. Gene immunotherapy of chronic lymphocytic leukemia: a phase I study of intranodally injected adenovirus expressing a chimeric CD154 molecule. Cancer resea
72.Predina JD, Keating J, Venegas O, Nims S, Singhal S. Neoadjuvant intratumoral immuno-gene therapy for non-small cell lung cancer. Discovery medicine. 2016;21:275-281.
73.Schiza A, Wenthe J, Mangsbo S, Eriksson E, Nilsson A, Totterman TH, et al. Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. Journal of
74.Garcia-Carbonero R, Salazar R, Duran I, Osman-Garcia I, Paz-Ares L, Bozada JM, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. Journal for i
75.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083-2092.
76.Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, et al. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172). J Infect Dis.
77.Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1
78.Smaill F, Jeyanathan M, Smieja M, Medina MF, Thanthrige-Don N, Zganiacz A, et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl
79.Diaz CM, Chiappori A, Aurisicchio L, Bagchi A, Clark J, Dubey S, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors.
80.Kallel H, Kamen AA. Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials. Biotechnol J. 2015;10:741-747.
81.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med. 2008;205:2717-2725.
82.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7-12.
83.Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccin Immunother. 2014;10:2875-2884.
84.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91:4407-4411.
85.Xin KQ, Jounai N, Someya K, Honma K, Mizuguchi H, Naganawa S, et al. Prime-boost vaccination with plasmid DNA and a chimeric adenovirus type 5 vector with type 35 fiber induces protective immunity against HIV. Gene Ther. 2005;12:1769
86.Gao GP, Yang Y, Wilson JM. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934-8943.
87.Okada N, Iiyama S, Okada Y, Mizuguchi H, Hayakawa T, Nakagawa S, et al. Immunological properties and vaccine efficacy of murine dendritic cells simultaneously expressing melanoma-associated antigen and interleukin-12. Cancer Gene The
88.Salgado CD, Kilby JM. Retroviruses and other latent viruses: the deadliest of pathogens are not necessarily the best candidates for bioterrorism. Journal of the South Carolina Medical Association. 2009;105:104-106.
89.Jacome A, Navarro S, Rio P, Yanez RM, Gonzalez-Murillo A, Lozano ML, et al. Lentiviral-mediated genetic correction of hematopoietic and mesenchymal progenitor cells from Fanconi anemia patients. Molecular therapy : the journal of the
90.Yasutsugu Suzuki and Youichi Suzuki (July 20th 2011). Gene Regulatable Lentiviral Vector System, Viral Gene Therapy Ke Xu, IntechOpen, DOI: 10.5772/18155.
91.Borsotti C, Borroni E, Follenzi A. Lentiviral vector interactions with the host cell. Curr Opin Virol. 2016;21:102-108.
92.Rossetti M, Gregori S, Hauben E, Brown BD, Sergi LS, Naldini L, et al. HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells. Hum Gene Ther. 20
93.Wang CX, Torbett BE. Role of the mammalian target of rapamycin pathway in lentiviral vector transduction of hematopoietic stem cells. Curr Opin Hematol. 2015;22:302-308.
94.Merlin S, Cannizzo ES, Borroni E, Bruscaggin V, Schinco P, Tulalamba W, et al. A Novel Platform for Immune Tolerance Induction in Hemophilia A Mice. Mol Ther. 2017;25:1815-1830.
95.Merlin S, Follenzi A. Transcriptional Targeting and MicroRNA Regulation of Lentiviral Vectors. Mol Ther Methods Clin Dev. 2019;12:223-232.
96.Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529-1541.
97.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318-322.
98.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818-823.
99.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158.
100.Sessa M, Lorioli L, Fumagalli F, Acquati S, Redaelli D, Baldoli C, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476-487.
101.Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151.
102.Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V, Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172:1582-1587.
103.Lopes L, Dewannieux M, Gileadi U, Bailey R, Ikeda Y, Whittaker C, et al. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J Virol. 2008;82:86-95.
104.Bobisse S, Rondina M, Merlo A, Tisato V, Mandruzzato S, Amendola M, et al. Reprogramming T lymphocytes for melanoma adoptive immunotherapy by T-cell receptor gene transfer with lentiviral vectors. Cancer Res. 2009;69:9385-9394.
105.Iglesias MC, Mollier K, Beignon AS, Souque P, Adotevi O, Lemonnier F, et al. Lentiviral vectors encoding HIV-1 polyepitopes induce broad CTL responses in vivo. Mol Ther. 2007;15:1203-1210.
106.Dai B, Yang L, Yang H, Hu B, Baltimore D, Wang P. HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells. Proc Natl Acad Sci U S A. 2009;106:20382-20387.
107.Lemiale F, Asefa B, Ye D, Chen C, Korokhov N, Humeau L. An HIV-based lentiviral vector as HIV vaccine candidate: Immunogenic characterization. Vaccine. 2010;28:1952-1961.
108.Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, et al. Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J Virol. 2003;77:5902-5910.
109.Pistello M, Bonci F, Zabogli E, Conti F, Freer G, Maggi F, et al. Env-expressing autologous T lymphocytes induce neutralizing antibody and afford marked protection against feline immunodeficiency virus. J Virol. 2010;84:3845-3856.
110.Chiuppesi F, Vannucci L, De Luca A, Lai M, Matteoli B, Freer G, et al. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J Virol. 2012;86:6563-6574.
111.Norton TD, Zhen A, Tada T, Kim J, Kitchen S, Landau NR. Lentiviral Vector-Based Dendritic Cell Vaccine Suppresses HIV Replication in Humanized Mice. Mol Ther. 2019;27:960-973.
112.Wee EG, Ondondo B, Berglund P, Archer J, McMichael AJ, Baltimore D, et al. HIV-1 Conserved Mosaics Delivered by Regimens with Integration-Deficient DC-Targeting Lentiviral Vector Induce Robust T Cells. Mol Ther. 2017;25:494-503.
113.Pinschewer DD. Virally vectored vaccine delivery: medical needs, mechanisms, advantages and challenges. Swiss Med Wkly. 2017;147:w14465.
114.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465-1468.
115.Tagawa ST, Lee P, Snively J, Boswell W, Ounpraseuth S, Lee S, et al. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer. 2003;98:144-154.
116.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745-1749.
117.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90:11478-11482.
118.Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother. 2017;13:2837-2848.
119.Hobernik D, Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018;19.
120.Cui Z. DNA vaccine. Adv Genet. 2005;54:257-289.
121.Colluru VT, Johnson LE, Olson BM, McNeel DG. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol. 2016;34:193-204.
122.Boyle JS, Silva A, Brady JL, Lew AM. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci U S A. 1997;94:14626-14631.
123.Weber R, Bossart W, Cone R, Luethy R, Moelling K. Phase I clinical trial with HIV-1 gp160 plasmid vaccine in HIV-1-infected asymptomatic subjects. Eur J Clin Microbiol Infect Dis. 2001;20:800-803.
124.Fidler S, Stohr W, Pace M, Dorrell L, Lever A, Pett S, et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet. 2020;395:888-898.
125.Spearman P, Mulligan M, Anderson EJ, Shane AL, Stephens K, Gibson T, et al. A phase 1, randomized, controlled dose-escalation study of EP-1300 polyepitope DNA vaccine against Plasmodium falciparum malaria administered via electroporation. Vaccine. 2016;34:5571-5578.
126.Klencke B, Matijevic M, Urban RG, Lathey JL, Hedley ML, Berry M, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin Cancer Res. 2002;8:1028-1037.
127.Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, et al. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 2002;62:5845-5852.
128.Norell H, Poschke I, Charo J, Wei WZ, Erskine C, Piechocki MP, et al. Vaccination with a plasmid DNA encoding HER-2/neu together with low doses of GM-CSF and IL-2 in patients with metastatic breast carcinoma: a pilot clinical trial. J Transl Med. 2010;8:53.
129.Staff C, Mozaffari F, Haller BK, Wahren B, Liljefors M. A Phase I safety study of plasmid DNA immunization targeting carcinoembryonic antigen in colorectal cancer patients. Vaccine. 2011;29:6817-6822.
130.Conry RM, Curiel DT, Strong TV, Moore SE, Allen KO, Barlow DL, et al. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clin Cancer Res. 2002;8:2782-2787.
131.Ginsberg BA, Gallardo HF, Rasalan TS, Adamow M, Mu Z, Tandon S, et al. Immunologic response to xenogeneic gp100 DNA in melanoma patients: comparison of particle-mediated epidermal delivery with intramuscular injection. Clin Cancer Res. 2010;16:4057-4065.
132.Yuan J, Ku GY, Gallardo HF, Orlandi F, Manukian G, Rasalan TS, et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun. 2009;9:5.
133.Wolchok JD, Yuan J, Houghton AN, Gallardo HF, Rasalan TS, Wang J, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044-2050.
134.Triozzi PL, Aldrich W, Allen KO, Carlisle RR, LoBuglio AF, Conry RM. Phase I study of a plasmid DNA vaccine encoding MART-1 in patients with resected melanoma at risk for relapse. J Immunother. 2005;28:382-388.
135.Weber J, Boswell W, Smith J, Hersh E, Snively J, Diaz M, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215-223.
136.Dangoor A, Lorigan P, Keilholz U, Schadendorf D, Harris A, Ottensmeier C, et al. Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine. Cancer Immunol Immunother. 2010;59:863-873.
137.Cassaday RD, Sondel PM, King DM, Macklin MD, Gan J, Warner TF, et al. A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Clin Cancer Res. 2007;13:540-549.
138.Nabel GJ, Gordon D, Bishop DK, Nickoloff BJ, Yang ZY, Aruga A, et al. Immune response in human melanoma after transfer of an allogeneic class I major histocompatibility complex gene with DNA-liposome complexes. Proc Natl Acad Sci U S A. 1996;93:15388-15393.
139.Nemunaitis J, Meyers T, Senzer N, Cunningham C, West H, Vallieres E, et al. Phase I Trial of sequential administration of recombinant DNA and adenovirus expressing L523S protein in early stage non-small-cell lung cancer. Mol Ther. 2006;13:1185-1191.
140.Hovav AH, Panas MW, Rahman S, Sircar P, Gillard G, Cayabyab MJ, et al. Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes. J Immunol. 2007;179:6725-6733.
141.Pollard C, De Koker S, Saelens X, Vanham G, Grooten J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol Med. 2013;19:705-713.
142.Iavarone C, O’Hagan D T, Yu D, Delahaye NF, Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16:871-881.
143.De Beuckelaer A, Grooten J, De Koker S. Type I Interferons Modulate CD8(+) T Cell Immunity to mRNA Vaccines. Trends Mol Med. 2017;23:216-226.
144.Cruz CC, Suthar MS, Montgomery SA, Shabman R, Simmons J, Johnston RE, et al. Modulation of type I IFN induction by a virulence determinant within the alphavirus nsP1 protein. Virology. 2010;399:1-10.
145.Maruggi G, Shaw CA, Otten GR, Mason PW, Beard CW. Engineered alphavirus replicon vaccines based on known attenuated viral mutants show limited effects on immunogenicity. Virology. 2013;447:254-264.
146.Kramps T, Elbers K. Introduction to RNA Vaccines. Methods Mol Biol. 2017;1499:1-11.
147.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488-495.
148.Scheel B, Aulwurm S, Probst J, Stitz L, Hoerr I, Rammensee HG, et al. Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur J Immunol. 2006;36:2807-2816.
149.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, et al. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685-696.
150.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396-401.
151.Sebastian M, Papachristofilou A, Weiss C, Fruh M, Cathomas R, Hilbe W, et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive(R)) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer. 2014;14:748.
152.Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498-507.
153.Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31:180-188.
154.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther. 2011;19:990-999.
155.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, et al. Long-term survival correlates with immunological responses in renal cell carcinoma patients treated with mRNA-based immunotherapy. Oncoimmunology. 2016;5:e1108511.
156.Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511-1520.
157.Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol Ther. 2017;25:1316-1327.
158.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523.
159.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970-971.
160.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020.
161.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020.
162.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020.
163.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260-1263.
164.Pan RY, Chung WH, Chu MT, Chen SJ, Chen HC, Zheng L, et al. Recent Development and Clinical Application of Cancer Vaccine: Targeting Neoantigens. J Immunol Res. 2018;2018:4325874.
165.Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol. 2011;5:150-155.
166.di Pietro A, Tosti G, Ferrucci PF, Testori A. Oncophage: step to the future for vaccine therapy in melanoma. Expert Opin Biol Ther. 2008;8:1973-1984.
167.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520-3526.
168.Schmitz-Winnenthal FH, Hohmann N, Niethammer AG, Friedrich T, Lubenau H, Springer M, et al. Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: A randomized, placebo-controlled, phase 1 trial. Oncoimmunology. 2015;4:e1001217.
169.Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59-68.
170.Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K, Maksymiuk A, et al. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer. 2010;11:391-395.
171.Eton O, Ross MI, East MJ, Mansfield PF, Papadopoulos N, Ellerhorst JA, et al. Autologous tumor-derived heat-shock protein peptide complex-96 (HSPPC-96) in patients with metastatic melanoma. J Transl Med. 2010;8:9.
172.Schirrmacher V. Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol Immunother. 2005;54:587-598.
173.Schreiber S, Kampgen E, Wagner E, Pirkhammer D, Trcka J, Korschan H, et al. Immunotherapy of metastatic malignant melanoma by a vaccine consisting of autologous interleukin 2-transfected cancer cells: outcome of a phase I study. Hum Gene Ther. 1999;10:983-993.
174.Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894-1900.
175.Kusumoto M, Umeda S, Ikubo A, Aoki Y, Tawfik O, Oben R, et al. Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF gene. Cancer Immunol Immunother. 2001;50:373-381.
176.Butterfield LH, Comin-Anduix B, Vujanovic L, Lee Y, Dissette VB, Yang JQ, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008;31:294-309.
177.Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, et al. Intravesical rAd-IFNalpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol. 2017;35:3410-3416.
178.Navai N, Benedict WF, Zhang G, Abraham A, Ainslie N, Shah JB, et al. Phase 1b Trial to Evaluate Tissue Response to a Second Dose of Intravesical Recombinant Adenoviral Interferon alpha2b Formulated in Syn3 for Failures of Bacillus Calmette-Guerin (BCG) Therapy in Nonmuscle Invasive Bladder Cancer. Ann Surg Oncol. 2016;23:4110-4114.
179.Dinney CP, Fisher MB, Navai N, O’Donnell MA, Cutler D, Abraham A, et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J Urol. 2013;190:850-856.
180.Morse MA, Chaudhry A, Gabitzsch ES, Hobeika AC, Osada T, Clay TM, et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother. 2013;62:1293-1301.
181.Butterfield LH, Economou JS, Gamblin TC, Geller DA. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med. 2014;12:86.
182.Gavazza A, Lubas G, Fridman A, Peruzzi D, Impellizeri JA, Luberto L, et al. Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Hum Gene Ther. 2013;24:728-738.
183.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572-576.
184.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803-808.




