CAR-T immunotherapy and cancer therapy
Cell therapy is a kind of medicine aiming to cure disease or alleviate disease symptoms via direct infusion or transplantation of cells, which can be autologous or allogeneic. With several decades’ development and optimization, immuno-oncology cells (such as T cells, nature killer
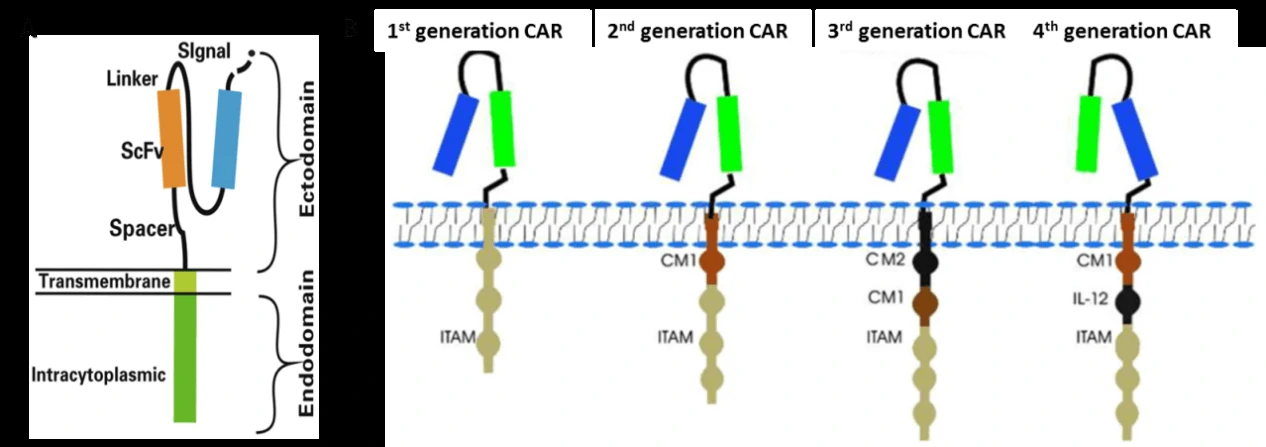
Chimeric antigen receptor (CAR)-modified T cells (CAR-T) are T cells genetically engineered to express CAR (Fig. 2A) [2,3], which can specifically recognize their target antigen via the scFv binding domain of CAR, resulting in T cell activation to specifically target and destroy tumor cells [3-5]. To date, four generations of CAR have been developed according to the structure of the endodomain (Fig. 2B) [3]. Due to little ability to generate enough interleukin-2 (IL-2), the 1st generation CAR-T cells (such as Ag-specific CD3ζ (MFEζ)-CAR-T cells, alpha-folate receptor (FR) -CAR-T cells, CE7R-CAR-T cells, scFv(G250)-CAR-T cells, GD2- CAR-T cells and CD10- CAR-T cells) benefitted substantially from the combination of cytokines [6], and were used for the treatment of different tumors [7-10]. However, most of the studies using the 1st generation CAR-T cells did not show very satisfactory results because of the inadequate proliferation, cytotoxicity, and insufficient secreted cytokines in vivo. To overcome these shortcomings, the 2nd generation CAR-T cells were designed by adding intracellular signaling domains from various co-stimulatory protein receptors to the cytoplasmic tail of the CARs, [11-13]. The 2nd generation CAR-T cells, such as scFvCD19-CD137-CD3-CAR-T cells, MOv19-BBζ-CAR-T cells and scFvCD19-CD28-CD3ζ-CAR-T cells displayed better curative effects on B cell malignancies [12,14]. On the basis of the 2nd generation CAR-T cells, the 3rd generation CAR-T cells were produced with the addition of multiple signaling domains, such as CD3ζ-CD28-OX40 or CD3ζ-CD28-41BB, to promote cytokine production and killing ability. The 3rd generation CAR-T cells, such as CD20-CD28-CD137-CD3ζ-CAR-T cells and HER2-CAR-T cells were applied for the treatment of lymphoma and colon cancer, but with no better outcomes than the 2nd generation CAR-T cells, and the reasons need to be further studied [15,16]. Based on the 2nd generation CAR-T cells, the 4th generation CAR-T cells were obtained with the addition of IL-12, and can mediate T cell redirected for universal cytokine-mediated killing (TRUCKs). TRUCK T cells show great outcomes in disease therapy by augmenting T-cell activation, and also activating and recruiting innate immune cells to destroy the antigen-negative cancer cells in the targeted lesion, and can also be used for the therapy of viral infections, metabolic disorders and auto-immune diseases [17]. Overall, these successive generations of CAR-T cells have yielded remarkable efficacy in several types of cancer or tumor therapy, and some of them have been translated into clinical trials with few side effects (Table 1) [18].
| Antigen | Disease | In vitro, in vivo, in preclinical or in clinical trials | NCT/Reference |
| CD19 | haematologic malignancies | clinical trials | [19-22] |
| CD20 | haematologic malignancies | clinical trials | [23,24] |
| TRAIL receptor 1 | Lymphoma | In vitro | [25] |
| Kappa | Lymphoma | clinical trials | NCT00881920 |
| CD22 | follicular lymphoma, non-Hodgkin’s lymphoma | clinical trials | NCT02315612 |
| HA-1 H | Leukaemia | In vitro | [26] |
| NKG2D | Leukaemia | clinical trials | NCT02203825 |
| FAP | B cell chronic lymphocytic leukaemia | clinical trials | NCT01722149 |
| ROR1 | chronic lymphocytic leukaemia | clinical trials | NCT02194374 |
| CD138 | multiple myeloma | clinical trials | NCT01886976 |
| NY-ESO-1 | multiple myeloma | In vitro | [27] |
| Lewis Y | multiple myeloma | clinical trials | NCT01716364 |
| HER2 | Osteosarcoma | In vitro | [28] |
| HER2 | Breast cancer | In vitro | [29] |
| HER2 | Sarcoma | Clinical trial | NCT00902044 |
| HER2 | Metastatic cancer | Clinical trial | NCT00924287 |
| HER2 | Glioblastoma | Clinical trial | NCT01109095 |
| HER2 | Solid tumors | Clinical trial | NCT01935843 |
| CEA | Colorectal cancer | In vivo | [30] |
| CEA | Colorectal cancer | Clinical trial | NCT00673322 |
| CEA | Breast cancer | Clinical trial | NCT00673829 |
| CEA | Liver metastases | Clinical trial | NCT01373047 |
| CEA | Metastatic cancers | Clinical trial | NCT01723306 |
| CSPG4 | Melanoma, breast carcinoma | In vivo | [31] |
| EphA2 | Glioblastoma | In vivo | [32] |
| FR | Ovarian cancer | In vivo | [33] |
| IL-11Rα | Osteosarcoma | In vivo | [34] |
| IL-13Rα2 | Glioblastoma | Preclinical trial | [35] |
| IL-13Rα2 | Malignant glioma | Clinical trial | NCT02208362 |
| IL-13R | Glioma | Preclinical trial | [36] |
| CD171 | Neuroblastoma | Clinical trial | NCT02311621 |
| EGFR | Advanced EGFR-positive solid tumors | Clinical trial | NCT01869166 |
| EGFR | Advanced glioma | Clinical trial | NCT02331693 |




