The Landscape of Neutralizing antibodies (NAb): Production, Mechanisms of Action (MOA), FDA approved-antibodies, and Functional assay
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

Table of Contents

FDA approved neutralizing antibodies (NAb) and our products
1. SARS-CoV-2 neutralizing antibodies (NAb)
SARS-CoV-2, the novel coronavirus responsible for the ongoing COVID-19 pandemic, has been spreading rampantly. The global scientific community has responded rapidly to understand immune correlates of protection to develop vaccines and immunotherapeutic against the virus. The RBD of spike plays a critical role in the very first step of the virus life cycle. The neutralizing antibodies (nAbs) that target the receptor binding domain (RBD) of viral spike (S) glycoprotein3.
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Bamlanivimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike therapeutic antibody |
Bamlanivimab |
NA |
SARS-CoV-2 Spike |
Whole mAb |
||
Pre-Made Casirivimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Casirivimab |
NA |
SARS-CoV-2Spike RBD |
Whole mAb |
||
Pre-Made Cilgavimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Cilgavimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Etesevimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Etesevimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Imdevimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Imdevimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Sotrovimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Sotrovimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Tixagevimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Tixagevimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Adintrevimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike therapeutic antibody |
Adintrevimab |
NA |
SARS-CoV-2 Spike |
Whole mAb |
||
Pre-Made Amubarvimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Amubarvimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Beludavimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Beludavimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Enuzovimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Enuzovimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Lomtegovimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Lomtegovimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Rimteravimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Rimteravimab |
NA |
SARS-CoV-2 Spike RBD |
Mixed Nanobody (VHH-CH2-CH3 dimer) |
||
Pre-Made Romlusevimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Romlusevimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Upanovimab biosimilar, Whole Mab: Anti-SARS-CoV-2 Spike RBD therapeutic antibody |
Upanovimab |
NA |
SARS-CoV-2 Spike RBD |
Whole mAb |
||
Pre-Made Ensovibep biosimilar, Recombinant Protein: Recombinant therapeutic protein targeting Severe?acute?respiratory syndrome?coronavirus?2 RBD |
ensovibep |
NA |
Severe?acute?respiratory syndrome?coronavirus?2 RBD |
Recombinant Protein |
2. Human Immunodeficiency Virus (HIV) neutralizing antibodies (NAb)
The glycoprotein (gp) 120 subunit is an important part of the envelope spikes that decorate the surface of HIV-1 and a major target for neutralizing antibodies.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Elipovimab biosimilar, Whole Mab: Anti-HIV-1 gp120 therapeutic antibody |
Elipovimab |
HIV-1 gp120 |
Whole mAb |
||
Pre-Made Suvizumab biosimilar, Whole Mab: Anti-HIV-1 gp120 therapeutic antibody |
Suvizumab |
HIV-1 gp120 |
Whole mAb |
||
Pre-Made Teropavimab biosimilar, Whole Mab: Anti-HIV-1 gp120 CD4bs therapeutic antibody |
Teropavimab |
HIV-1 gp120 CD4bs |
Whole mAb |
||
Pre-Made Alvircept Sudotox biosimilar, Fusion Protein: Recombinant therapeutic protein targeting HIV-1 gp120 fused with Pseudomonas aeruginosa exotoxin A |
alvircept sudotox |
HIV-1 gp120 |
Fusion Protein |
3. Hepatitis B virus (HBV) neutralizing antibodies (NAb)
Hepatitis B virus (HBV) surface antigen (HBsAg) is considered to be the most important target for the diagnosis and immune prophylaxis of HBV infection. HBsAg-specific monoclonal antibodies (MAbs) are extensively used for studying the complex structure of the HBsAg, mapping the neutralizing epitopes and development of HBV diagnostic tests.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Lenvervimab biosimilar, Whole Mab: Anti-HBV therapeutic antibody |
Lenvervimab |
HBV |
Whole mAb |
||
Pre-Made Tuvirumab Biosimilar, Whole Mab: Anti-Hbv (Hepatitis B Virus) [Hepatitis B Virus] therapeutic antibody |
tuvirumab |
HBV (hepatitis B virus) [Hepatitis B virus] |
Whole mAb |
||
Pre-Made Exbivirumab Biosimilar, Whole Mab: Anti-Hbv (Hepatitis B Virus) Surface Antigen (Hbsag) [Hepatitis B Virus] therapeutic antibody |
exbivirumab |
HBV (hepatitis B virus) surface antigen (HBsAg) [Hepatitis B virus] |
Whole mAb |
||
Pre-Made Libivirumab Biosimilar, Whole Mab: Anti-Hbv (Hepatitis B Virus) Surface Antigen (Hbsag) [Hepatitis B Virus] therapeutic antibody |
libivirumab |
HBV (hepatitis B virus) surface antigen (HBsAg) [Hepatitis B virus] |
Whole mAb |
4. Rabies virus neutralizing antibodies (NAb)
Rabies virus is the causative agent of the rabies. The Rabies Virus Spike Glycoproteins are the important protein responsible for the virus entry and the major target of neutralizing antibodies. GeneMedi offers the Rabies Virus Spike Glycoprotein G, Rabies Virus Surface Glycoprotein 4 (gp4) Epitope 1, Rabies Virus Strain ERA GP Ectodomain Epitope G-II, Rabies Virus Spike Glycoprotein G, Rabies Virus Strain ERA GP Ectodomain Epitope G-III, RV Antigenic Site III, RV Antigenic Site III-specific monoclonal antibodies (MAbs) for therapeutic application and development of Rabies virus diagnostic tests.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Docaravimab biosimilar, Whole mAb (Mouse): Anti-Rabies Virus Strain ERA GP Ectodomain Epitope G-III therapeutic antibody |
Docaravimab |
Rabies Virus Strain ERA GP Ectodomain Epitope G-III |
Whole mAb (Mouse) |
||
Pre-Made Foravirumab biosimilar, Whole Mab: Anti-RV Antigenic Site III therapeutic antibody |
Foravirumab |
RV Antigenic Site III |
Whole mAb |
||
Pre-Made Miromavimab biosimilar, Whole mAb (Mouse): Anti-Rabies Virus Strain ERA GP Ectodomain Epitope G-II therapeutic antibody |
Miromavimab |
Rabies Virus Strain ERA GP Ectodomain Epitope G-II |
Whole mAb (Mouse) |
||
Pre-Made Rafivirumab biosimilar, Whole Mab: Anti-RV Antigenic Site III therapeutic antibody |
Rafivirumab |
RV Antigenic Site III |
Whole mAb |
||
Pre-Made Mazorelvimab biosimilar, Whole Mab: Anti-Rabies Virus Spike Glycoprotein G therapeutic antibody |
Mazorelvimab |
Rabies Virus Spike Glycoprotein G |
Whole mAb |
||
Pre-Made Ormutivimab biosimilar, Whole Mab: Anti-Rabies Virus Surface Glycoprotein 4 (gp4) Epitope 1 therapeutic antibody |
Ormutivimab |
Rabies Virus Surface Glycoprotein 4 (gp4) Epitope 1 |
Whole mAb |
||
Pre-Made Zamerovimab biosimilar, Whole Mab: Anti-Rabies Virus Spike Glycoprotein G therapeutic antibody |
Zamerovimab |
Rabies Virus Spike Glycoprotein G |
Whole mAb |
5. Respiratory syncytial virus (RSV) neutralizing antibodies (NAb)
Respiratory syncytial virus (RSV) enters into the host cells through the type I fusion (F) glycoprotein. Neutralizing monoclonal antibodies, such as Suptavumab, Palivizumab, Nirsevimab, Motavizumab, Gontivimab, and felvizumab target the major antigenic sites on the RSV F glycoprotein and neutralize the entry process.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Gontivimab biosimilar, Bispecific Single Domains (VH-VH'-VH¡¯): Anti-RSV gpF therapeutic antibody |
Gontivimab |
RSV gpF |
Bispecific Single Domains (VH-VH'-VH¡¯) |
||
Pre-Made Motavizumab biosimilar, Whole Mab: Anti-RSV gpF therapeutic antibody |
Motavizumab |
RSV gpF |
Whole mAb |
||
Pre-Made Nirsevimab biosimilar, Whole Mab: Anti-RSV therapeutic antibody |
Nirsevimab |
RSV |
Whole mAb |
||
Pre-Made Palivizumab biosimilar, Whole Mab: Anti-RSV gpF therapeutic antibody |
Palivizumab |
RSV gpF |
Whole mAb |
||
Pre-Made Suptavumab biosimilar, Whole Mab: Anti-RSV gpF therapeutic antibody |
Suptavumab |
RSV gpF |
Whole mAb |
||
Pre-Made Felvizumab Biosimilar, Whole Mab: Anti-Rsv (Respiratory Syncytial Virus) Glycoprotein F [Respiratory Syncytial Virus] therapeutic antibody |
felvizumab |
RSV (respiratory syncytial virus) glycoprotein F [Respiratory syncytial virus] |
Whole mAb |
6. Zaire Ebola virus neutralizing antibodies (NAb)
Zaire Ebola virus belongs to the family Filoviridae, which are highly lethal and it outbreaks since emerging in 1967, including a sustained epidemic in West Africa from 2013 to 2015. Zaire Ebolavirus (EBOV) glycoprotein (GP) is now the key target of antibodies. GP is composed of ectodomain GP1 and a trans-membrane fusion domain GP2. GP1 and GP2 helps the virus to enter host cell through attachment, uptake, and fusion. GeneMedi offers the monoclonal antibodies (MAbs) against Zaire Ebolavirus GP, and Zaire Ebolavirus GP1 for therapeutic application and development of diagnostic tests.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Ansuvimab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP1 therapeutic antibody |
Ansuvimab |
Zaire Ebolavirus GP1 |
Whole mAb |
||
Pre-Made Atoltivimab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP therapeutic antibody |
Atoltivimab |
Zaire Ebolavirus GP |
Whole mAb |
||
Pre-Made Cosfroviximab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP therapeutic antibody |
Cosfroviximab |
Zaire Ebolavirus GP |
Whole mAb |
||
Pre-Made Larcaviximab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP therapeutic antibody |
Larcaviximab |
Zaire Ebolavirus GP |
Whole mAb |
||
Pre-Made Maftivimab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP therapeutic antibody |
Maftivimab |
Zaire Ebolavirus GP |
Whole mAb |
||
Pre-Made Odesivimab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP therapeutic antibody |
Odesivimab |
Zaire Ebolavirus GP |
Whole mAb |
||
Pre-Made Porgaviximab biosimilar, Whole Mab: Anti-Zaire Ebolavirus GP therapeutic antibody |
Porgaviximab |
Zaire Ebolavirus GP |
Whole mAb |
7. Influenza virus neutralizing antibodies (NAb)
Influenza is a transmissible acute respiratory disease caused by the influenza virus. The glycoprotein, hemagglutinin (HA) of influenza virus plays an important role during the viral infection. It helps the virus to bind the cell surface receptors, and transfer the genetic materials into the host cytoplasm through fusion. The neutralizing antibodies against the haemagglutinin (HA) offer the protection against influenza virus infection. GeneMedi offers the monoclonal antibodies (MAbs) against Influenza A HA, Influenza B Virus and Influenza A HA2 for therapeutic application and development of diagnostic tests.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Diridavumab biosimilar, Whole Mab: Anti-Influenza A HA2 therapeutic antibody |
Diridavumab |
Influenza A HA2 |
Whole mAb |
||
Pre-Made Firivumab biosimilar, Whole Mab: Anti-Influenza A HA therapeutic antibody |
Firivumab |
Influenza A HA |
Whole mAb |
||
Pre-Made Gedivumab biosimilar, Whole Mab: Anti-Influenza A HA therapeutic antibody |
Gedivumab |
Influenza A HA |
Whole mAb |
||
Pre-Made Lesofavumab biosimilar, Whole Mab: Anti-Influenza B Virus therapeutic antibody |
Lesofavumab |
Influenza B Virus |
Whole mAb |
||
Pre-Made Navivumab biosimilar, Whole Mab: Anti-Influenza A HA therapeutic antibody |
Navivumab |
Influenza A HA |
Whole mAb |
8. Human cytomegalovirus (HCMV) neutralizing antibodies (NAb)
Human cytomegalovirus (HCMV) causes life-threatening illness in immunosuppressed patients. HCMV envelope proteins viz. glycoprotein B (gB), gH, gL, gO and UL128/UL130/UL131A are essential for the fusion of virus with the host plasma membranes to enter into the host cell. The gB protein is the direct mediator of cell fusion and it requires gH/gL/gO protein complex for activation. HCMV gB or gH/gL proteins provoke HCMV neutralizing antibodies to prevent the entry of virus into both fibroblasts and epithelial cells. GeneMedi offers the monoclonal antibodies (MAbs) against envelope glycoprotein H (gH) and envelope glycoprotein B (gB) for therapeutic application and development of human cytomegalovirus diagnostic tests.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Regavirumab Biosimilar, Whole Mab: Anti-Hcmv (Human Cytomegalovirus) Envelope Glycoprotein B (Gb) [Human Betaherpesvirus 5] therapeutic antibody |
regavirumab |
HCMV (human cytomegalovirus) envelope glycoprotein B (gB) [Human betaherpesvirus 5] |
Whole mAb |
||
Pre-Made Sevirumab Biosimilar, Whole Mab: Anti-Hcmv (Human Cytomegalovirus) Envelope Glycoprotein H (Gh) [Human Betaherpesvirus 5] therapeutic antibody |
sevirumab |
HCMV (human cytomegalovirus) envelope glycoprotein H (gH) [Human betaherpesvirus 5] |
Whole mAb |
9. Human endogenous retroviral envelope protein HERV-W-Env neutralizing antibodies (NAb)
Temelimab (formerly called GNbAC1) is an immunoglobulin (Ig) G4 monoclonal antibody that targets the human endogenous retroviral envelope protein HERV-W-Env, shown to be associated with the pathogenesis of certain autoimmune disorders such as multiple sclerosis (MS) and type 1 diabetes mellitus (T1D). By neutralizing HERV-W-Env, temelimab could act as a disease-modifying therapy for these disorders. Hence, GeneMedi offers the monoclonal antibodies against MRSV envelope protein for therapeutic application and development of diagnostic tests.
10. Pseudomonas aeruginosa infections neutralizing antibodies (NAb)
Pseudomonas aeruginosa is a main source of health-care related infections, including pneumonia and infections associated with urinary tract, wounds, burns, and the bloodstream. P. aeruginosa invades the eukaryotic host cells through the type III secretion system (T3SS). This T3SS helps the bacteria to translocate effector proteins directly into host cells. Mammalian immune systems produce neutralizing antibodies (Ab) against T3SS proteins during infection to inhibit the Pseudomonas aeruginosa infections. Hence, GeneMedi offers the different kind of antibodies such as pegylated, Fab, and Bispecific mAb against the PcrV type III secretion system of Pseudomonas aeruginosa.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Gremubamab biosimilar, Bispecific mAb: Anti-PcrV type III secretion system; Polysaccharide synthesis locus (Pseudomonas) therapeutic antibody |
Gremubamab |
PcrV type III secretion system,Polysaccharide synthesis locus (Pseudomonas) |
Bispecific mAb |
||
Pre-Made Rivabazumab biosimilar, Fab: Anti-PcrV type III secretion system therapeutic antibody |
Rivabazumab |
PcrV type III secretion system |
Fab |
11. Measles virus (MeV) neutralizing antibodies (NAb)
Measles virus (MeV) causes an extremely transmissible respiratory disease and it has also been used as an oncolytic platform. MeV enter into the cells through the cellular receptors such as SLAM (signaling lymphocyte-activating molecule) and PVRL4 (poliovirus receptor-like 4) (nectin-4). Hence, the antibodies against PVRL4 could block measles virus infections. GeneMedi offers monoclonal antibodies against the PVRL4 to prevent the MeV infection.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Enfortumab biosimilar, Whole mAb ADC, Anti-PVRL4/NECTIN4 Antibody: Anti-EDSS1/LNIR/PRR4/nectin-4 therapeutic antibody |
Enfortumab |
PVRL4 |
Whole mAb ADC |
||
Pre-Made Enfortumab Vedotin Biosimilar, Whole Mab Adc, Anti-PVRL4/NECTIN4 Antibody: Anti-EDSS1/LNIR/PRR4/nectin-4 therapeutic antibody Drug Conjugate |
enfortumab vedotin |
PVRL4 |
Whole mAb ADC |
12. Anthrax protective antigen (PA) neutralizing antibodies (NAb)
Bacillus anthracis is the causative agent of anthrax. The protective antigen is an important element of the anthrax toxin. It helps bacteria to transfer the enzymatic components into the host cell, through the formation spanning pore on the membrane. antibiotics are effective at the early stages of anthrax, but antibiotics are no longer effective with the accumulation of anthrax toxin. Therefore, the anti-toxin antibody is the most promising approach to treat the anthrax. GeneMedi offers the monoclonal antibodies (MAbs) against anthrax protective antigen.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Obiltoxaximab biosimilar, Whole Mab: Anti-Anthrax Protective Antigen therapeutic antibody |
Obiltoxaximab |
Anthrax Protective Antigen |
Whole mAb |
||
Pre-Made Raxibacumab Biosimilar, Whole Mab: Anti-Anthrax Protective Antigen (Pa) [Bacillus Anthracis] therapeutic antibody |
raxibacumab |
anthrax protective antigen (PA) [Bacillus anthracis] |
Whole mAb |
13. Shiga toxins (Stxs) neutralizing antibodies (NAb)
Shiga toxins (Stxs) are cytotoxic proteins secreted by Shigella dysenteriae 1 and some serogroups of Escherichia coli (called Stx1 in E. coli). Shiga toxin producing E. coli (STEC) are foodborne pathogens that may colonize and damage the human colon to access to the bloodstream and damage the organs such as kidney and brain. Presently, there are no specific protective treatment against Stx intoxication, and it based on the rehydration therapy, and dialysis. The neutralization of Stx using neutralizing antibody is one of the promising approaches. Therefore, GeneMedi offers monoclonal antibody against Shiga Toxin Type 1 and Type 2.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Pritoxaximab biosimilar, Whole Mab: Anti-Shiga Toxin Type 1 therapeutic antibody |
Pritoxaximab |
Shiga Toxin Type 1 |
Whole mAb |
||
Pre-Made Setoxaximab biosimilar, Whole Mab: Anti-Shiga Toxin Type 2 therapeutic antibody |
Setoxaximab |
Shiga Toxin Type 2 |
Whole mAb |
||
Pre-Made Urtoxazumab Biosimilar, Whole Mab: Anti-E.Coli Shiga-Like Toxin 2 B Subunit therapeutic antibody |
urtoxazumab |
E.coli Shiga-like toxin 2 B subunit |
Whole mAb |
14. Toxin A neutralizing antibodies (NAb)
Clostridium difficile continues to be one of the most prevalent hospital-acquired bacterial infections in the developed world. Alternative approaches under investigation to combat the anaerobic Gram-positive bacteria include fecal transplantation therapy, vaccines, and antibody-based immunotherapies. Inhibitory antibodies that recognize the primary C. difficilevirulence factors, toxin A and toxin B, are the most popular passive immunotherapies under investigation.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Actoxumab biosimilar, Whole Mab: Anti-Toxin A therapeutic antibody |
Actoxumab |
Toxin A |
Whole mAb |
||
Pre-Made Atidortoxumab biosimilar, Whole Mab: Anti-Toxin A therapeutic antibody |
Atidortoxumab |
Toxin A |
Whole mAb |
||
Pre-Made Berlimatoxumab biosimilar, Whole Mab: Anti-Toxin A therapeutic antibody |
Berlimatoxumab |
Toxin A |
Whole mAb |
||
Pre-Made Bezlotoxumab biosimilar, Whole Mab: Anti-Toxin A therapeutic antibody |
Bezlotoxumab |
Toxin A |
Whole mAb |
||
Pre-Made Suvratoxumab biosimilar, Whole Mab: Anti-Toxin A therapeutic antibody |
Suvratoxumab |
Toxin A |
Whole mAb |
||
Pre-Made Tosatoxumab biosimilar, Whole Mab: Anti-Toxin A therapeutic antibody |
Tosatoxumab |
Toxin A |
Whole mAb |
15. Others neutralizing antibodies (NAb)
Monoclonal antibodies (mAbs) against bacterial infections are more effective way to treat the antibiotic resistant strains. The antibacterial mAbs have several advantages such as reducing the antibiotic toxicity, treatment time and disease complications. there are numerous mAb products are under development for a range of infectious diseases. Currently, GeneMedi offers mAbs against gram negative bacteria, Staphylococcus epidermidis, Staphylococcus aureus and so on.
Cat No. | Products Name (INN Index) | INN Name | Target | Format | Order |
|---|---|---|---|---|---|
Pre-Made Bentracimab biosimilar, Whole Mab: Anti-AR-C124910XX therapeutic antibody |
Bentracimab |
AR-C124910XX |
Whole mAb |
||
Pre-Made Omodenbamab biosimilar, Whole Mab: Anti-SpA therapeutic antibody |
Omodenbamab |
SpA |
Whole mAb |
||
Pre-Made Panobacumab biosimilar, Whole Mab: Anti-Serotype IATS O11 therapeutic antibody |
Panobacumab |
Serotype IATS O11 |
Whole mAb |
||
Pre-Made Tefibazumab Biosimilar, Whole Mab: Anti-Staphylococcus Aureus Fibrin-Binding Surface Epitope Clumping Factor A (Clfa) therapeutic antibody |
tefibazumab |
Staphylococcus aureus fibrin-binding surface epitope clumping factor A (ClfA) |
Whole mAb |
||
Pre-Made Edobacomab Biosimilar, Whole Mab: Anti-Endotoxin (Lipid A, Domain Of Lipopolyaccharide, Lps) [Gram Negative Bacteria] therapeutic antibody |
edobacomab |
endotoxin (lipid A, domain of lipopolyaccharide, LPS) [gram negative bacteria] |
Whole mAb |
||
Pre-Made Nebacumab Biosimilar, Whole Mab: Anti-Endotoxin (Lipid A, Domain Of Lipopolyaccharide, Lps) [Gram Negative Bacteria] therapeutic antibody |
nebacumab |
endotoxin (lipid A, domain of lipopolyaccharide, LPS) [gram negative bacteria] |
Whole mAb |
||
Pre-Made Pagibaximab Biosimilar, Whole Mab: Anti-Staphylococcus Epidermidis Lipoteichoic Acid therapeutic antibody |
pagibaximab |
Staphylococcus epidermidis lipoteichoic acid |
Whole mAb |
Introduction of neutralizing antibody (NAb)
Definition--What is neutralizing antibody (NAb)?
A neutralizing antibody (NAb) is an antibody that is responsible for defending cells from pathogens, which are organisms that cause disease. They are produced naturally by the body as part of its immune response, and their production is triggered by both infections and vaccinations against infections1. A neutralising antibody rapid test, such as COVID-19 neutralising antibody (Nab) assay development kit, can detect IgG antibodies against the full trimeric spike protein of the SARS-CoV-2 virus. Meaning it has the potential to play a vital supporting role out of the pandemic. A neutralizing antibody titer of 10 corresponded to 0.01 IU/ml, the level considered necessary for short-term protection.
Diffirernce between neutralizing antibody (NAb) and binding antibody
Neutralizing antibodies can be confused with binding antibodies (non neutralizing antibody), which are responsible for binding to a pathogen and alerting the immune system to its presence so white blood cells can be sent to destroy it. Neutralizing antibodies, although an integral part of the body’s immune response, serve a different purpose to binding antibodies1.
How to produce neutralizing antibody (NAb) (production)
Antibodies are made by B-cells in the bone marrow. Bone marrow has been long thought to be a hematopoietic organ. When B-cells are created, they begin to produce antibodies that will bind to specific antigens. Antigen-specific antibody producing, long-term lived plasma cells are largely found in the bone marrow, which contributes to humoral immune responses.
Affifinity maturation is based on the somatic mutation of the germline genes of immunoglobulin, a process called somatic hypermutation (SHM), which consists of point mutations that are performed by a protein called activation-induced cytosine deaminase (AID), resulting in a greater affifinity of antibodies or, in the worst cases, a decrease in affifinity.
This process is carried out in germinal centers (GCs), specialized microstructures formed in secondary lymphoid tissues upon infection or immunization, producing longlived plasma cells and memory B cells, which protect against reinfection. GCs are organized into two regions, the dark zone (DZ) and the light zone (LZ).
In the DZ, there are B cells with a high proliferation rate (called centroblasts) and SHM. The centroblasts then enter the LZ (now are called centrocytes), where they capture and process antigens present on follicular dendritic cells (FDC) and they subsequently present antigenic peptides to T follicular helper (Tfh) cells to in order receive critical survival signals and undergo selection. Moreover, FDCs can have the ability to retain the native antigen to carry out the previous process, in addition to producing cytokines, such as BAFF, that help in the survival of the B cell.
In the LZ class-switch recombination (CSR) also occurs, where the constant region of the heavy chain of the antibody is changed, allowing B cells to produce IgG antibodies, IgA or IgE. This process diversififies the effector functions of antibodies, e.g., IgG can activate NK cells and phagocytes to eliminate cells infected by pathogens. In the final stage of the germinal center process, the centrocytes exit the GC as memory B cells or high-affifinity antibody-secreting plasma cells, but the molecular mechanisms that underlie this process are not yet entirely clear.
In the case of a plasma cell, the key is the activation of the Blimp1 master regulator (Prdm1), which, among many other functions, helps to stop the expression of transcription factors, such as Pax5 and Bcl6, responsible for the maintenance of B cells and the germinal center. For memory B cells, there is no established master regulator; however, a greater expression of Bach2 is fundamental. Plasma cells reside in the bone marrow and constitutively secrete antibodies; they do not possess BCR receptors, are not reactivated with antigenic re-exposition, and are responsible for producing serum antibodies that can last years after infection or vaccination. On the other hand, memory B cells express BCR but do not secrete antibodies constitutively. When they meet again with the antigen, they can reactivate and form GCs to produce antibodies with a greater affifinity; moreover, these cells can give rise to plasma cells and reside in circulation or peripheral lymphoid tissue.
The development of B cells can be seen in Figure 1. The antigen is processed in a dendritic cell to ensure its presentation and to produce cytokines. The dendritic cell within the lymph node presents antigen by class II MHC to naive T-lymphocytes, which differentiate in an effector or memory T-cell. The B cell can recognize native, unprocessed antigens with its BCR, but to perform a more specific response, the antigen needs to enter a germ center to be in contact with a follicular T cell that has already been presented with it, as this allows for it to be presented to the B cell and for the germ center reaction to begin, thereby producing specific antibodies, in this case, those against SARS-CoV-2 2.
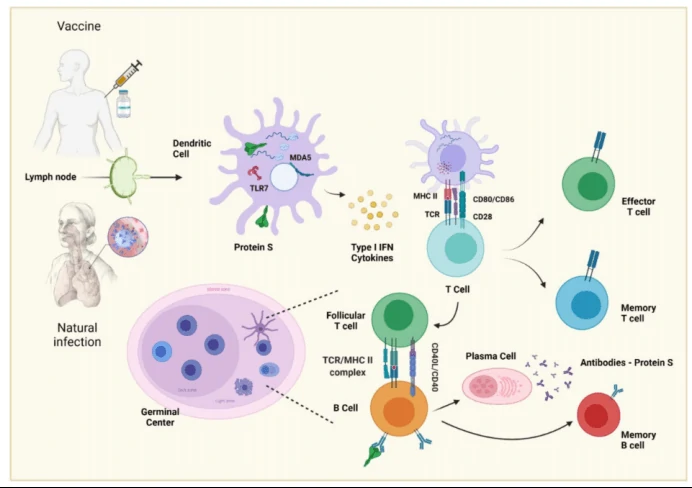
How neutralizing antibody (NAb) work (mechanisms of action, MOA)
Regarding neutralizing antibody activity, Neutralization can be achieved through four main mechanisms2:
(1)NAbs binding to viral surface proteins and blocking their interaction with the host cell receptor and infection;
(2)NAbs binding to viral protein epitopes that interact with host cell coreceptors that are key for viral infection;
(3)NAbs binding to viral epitopes that are not essential for host cell receptor binding but are necessary for conformational changes needed for membrane fusion. A variant of this mechanism would be the capability of NAbs to bind to proteins essential for host cell receptor binding, but NAbs are bound to distal epitopes of fusogenic proteins (internalized), preventing complete fusion. The above mechanisms can be classified as inhibition of the entry of the virus into host cells; other mechanisms may provide post-binding inhibition. Some viruses require internalization and a pH decrease to trigger conformational changes and to perform viral membrane fusion.
(4)The fourth mechanism of neutralization can occur once the virus are inside endosomes; the junction of the antibodies to viral surface proteins inhibits the necessary changes for the fusion of the viral membrane, causing neutralization. This last mechanism could target enveloped and naked virus particles, and it is a post-internalization neutralization.
Take “Interaction of SARS-CoV-2 with its receptors and neutralization mechanisms” as an example (Figure 2). S1 contains the receptor-binding domain (RBD) and directly binds to ACE2 to gain entry into host cells. There are four neutralizing mechanisms2: (1) NAbs bound to the receptor-binding protein (S) and block its interaction with ACE2; (2) the virion establishes contact between its binding protein and the receptor on the cell surface, and NAbs block subsequent steps, such as binding to a coreceptor; (3) the virion is about to fuse with the cell membrane, but NAbs are bound to proteins that are not essential for cell receptor binding but exert conformational changes that do not allow virus internalization with the cell membrane; (4) NAbs prevent the virion from merging its envelope with the vesicular membrane (endosome) and begin viral replication, and the binding of the antibody to the virus inhibits the conformational changes necessary for membrane fusion.
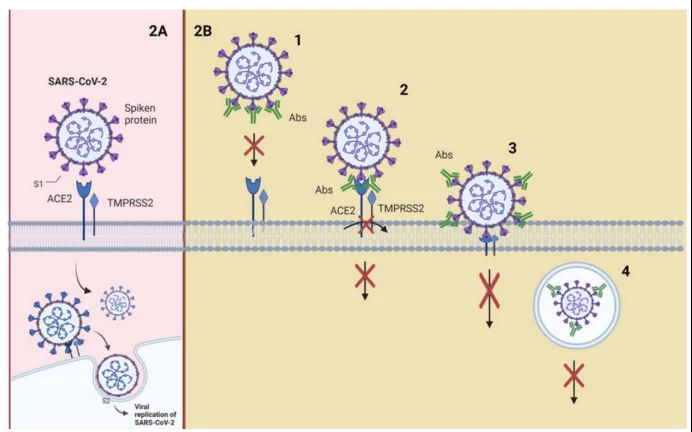
Functional assay for neutralizing antibodies (NAb) discovery--Pseudotype virus (psedovirus, PSV) based and Neutralization Assays
The globalization of the world’s economies, accompanied by increasing international travel, changing climates, altered human behaviour and demographics is leading to the emergence of different viral diseases, many of which are highly pathogenic and hence are considered of great public and animal health importance. To undertake basic research and therapeutic development, many of these viruses require handling by highly trained staff in BSL-3/4 facilities not readily available to the majority of the global R&D community. In order to circumvent the enhanced biosafety requirement, the development of nonpathogenic, replication-defective pseudotyped viruses is an effective and established solution to permit the study of many aspects of virus biology in a low containment biosafety level (BSL)-1/2 laboratory4.
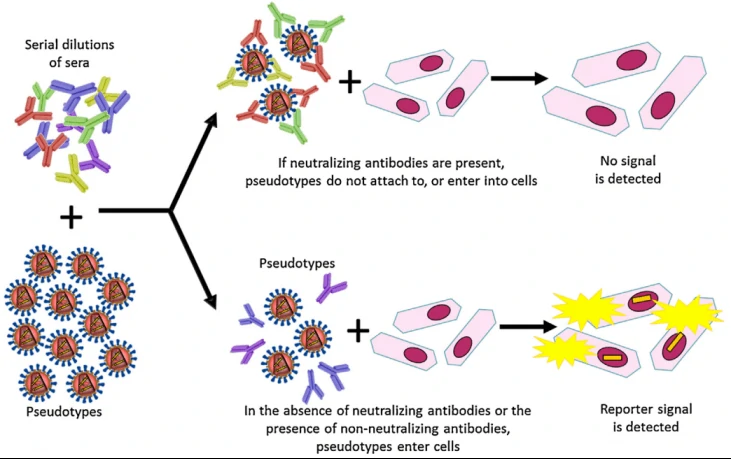
SARS-CoV-2 neutralizing antibody validated post download
–Nab discovery and vaccines evaluation through SARS-CoV-2 wildtype/mutant variants pseudovirus based neutralizing assay(PBNA) and Spike-ACE2 competition binding assay
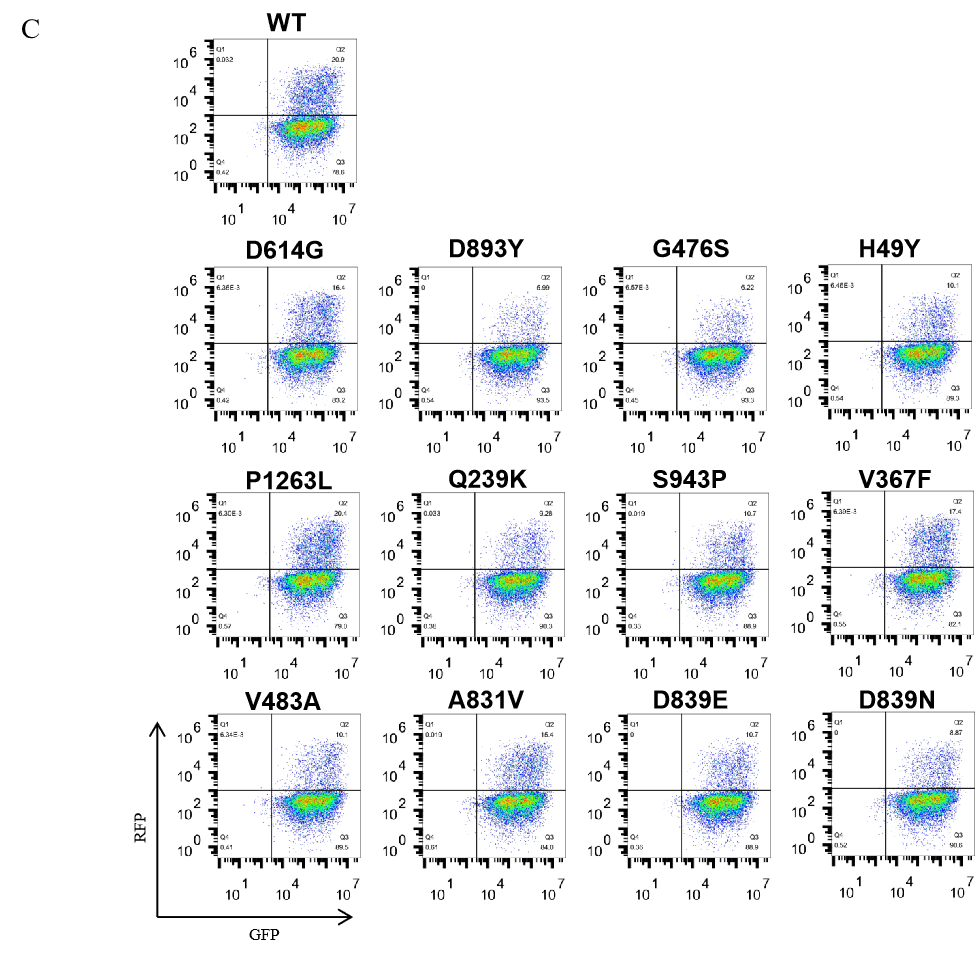
GeneMedi-SARS-CoV-2 WT and Spike Mutation Variants Pseudovirus (PSV) Based Cell Entry
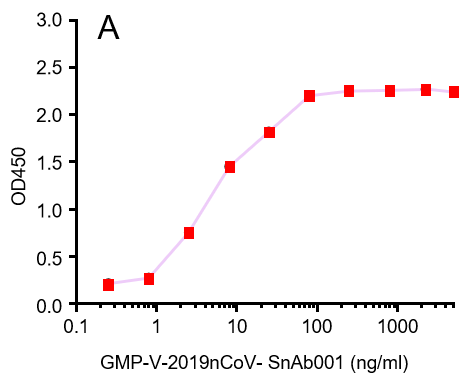
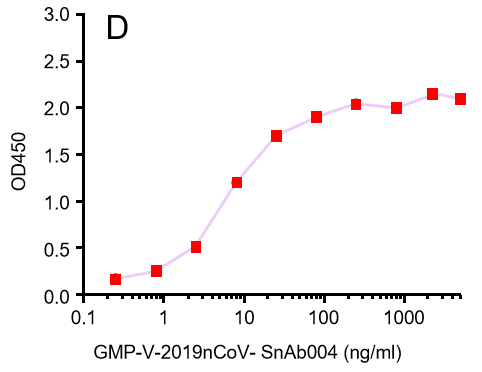
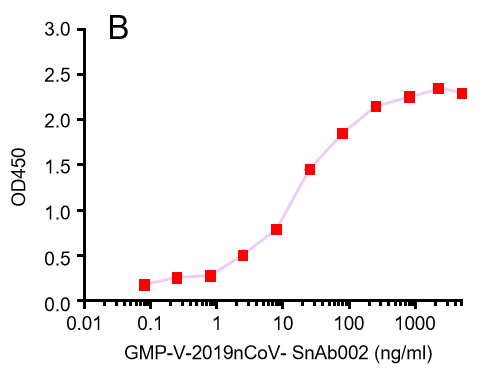
GeneMedi's anti-2019-nCoV Spike Neutralizing antibodies (Nabs) and Spike RBD protein binding validation
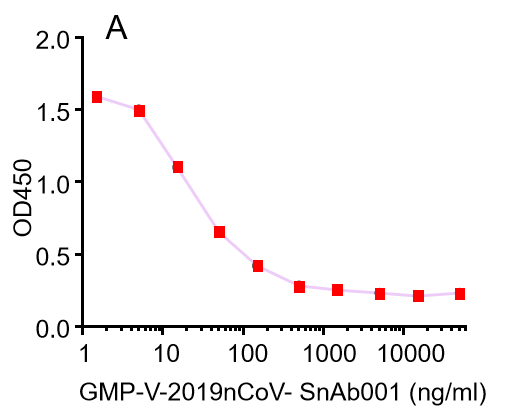
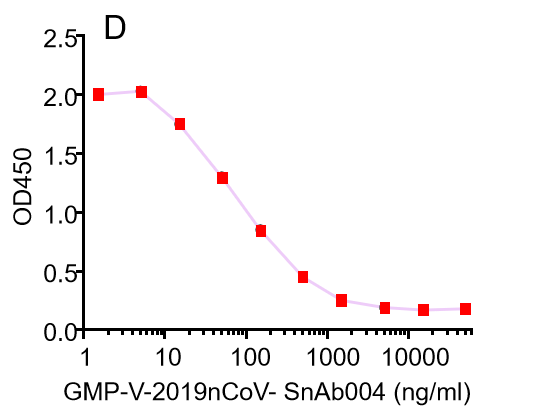
| Cat No. | Product | EC50 (ng/ml) |
| GMP-V-2019nCoV-SnAb001 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1) | 5 |
| GMP-V-2019nCoV-SnAb002 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM) | 18 |
| GMP-V-2019nCoV-SnAb003 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA) | 410 |
| GMP-V-2019nCoV-SnAb004 | Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1) | 6.8 |
| GMP-V-2019nCoV-SnAb005 | Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1) | 28 |
A.GMP-V-2019nCoV-SnAb001:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1)
B.GMP-V-2019nCoV-SnAb002:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM)
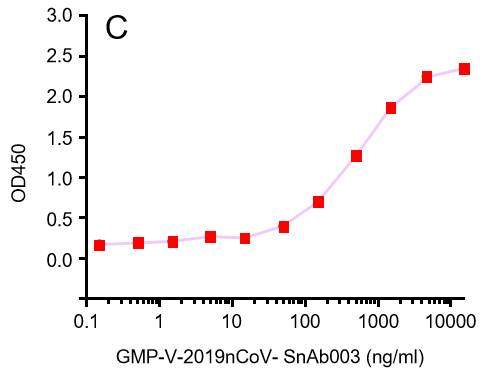
C.GMP-V-2019nCoV-SnAb003:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA)
D.GMP-V-2019nCoV-SnAb004:Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1)
E.GMP-V-2019nCoV-SnAb005:Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1)
GeneMedi's anti-2019-nCoV Spike Neutralizing antibodies (Nabs) competitive binding assay validation
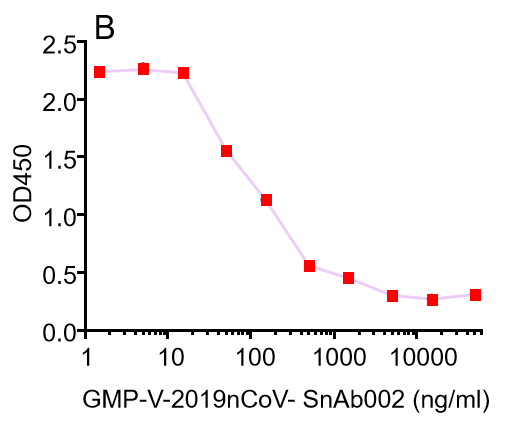
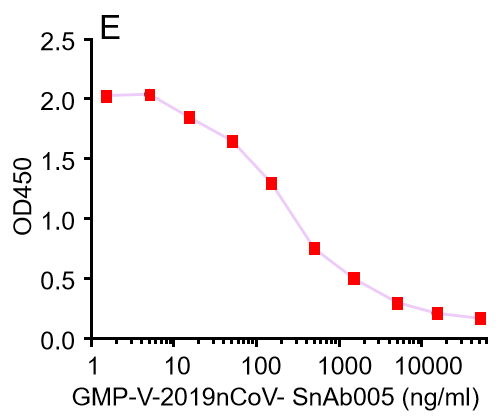
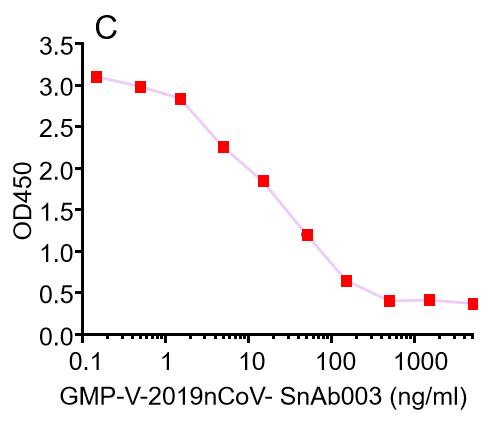
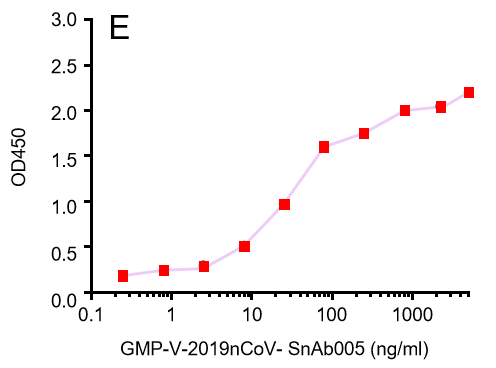
| Cat No. | Product | IC50 (ng/ml) |
| GMP-V-2019nCoV-SnAb001 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1) | 26.3 |
| GMP-V-2019nCoV-SnAb002 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM) | 84.2 |
| GMP-V-2019nCoV-SnAb003 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA) | 20.5 |
| GMP-V-2019nCoV-SnAb004 | Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1) | 81.9 |
| GMP-V-2019nCoV-SnAb005 | Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1) | 243 |
A.GMP-V-2019nCoV-SnAb001:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1)
B.GMP-V-2019nCoV-SnAb002:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM)
C.GMP-V-2019nCoV-SnAb003:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA)
D.GMP-V-2019nCoV-SnAb004:Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1)
E.GMP-V-2019nCoV-SnAb005:Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1)
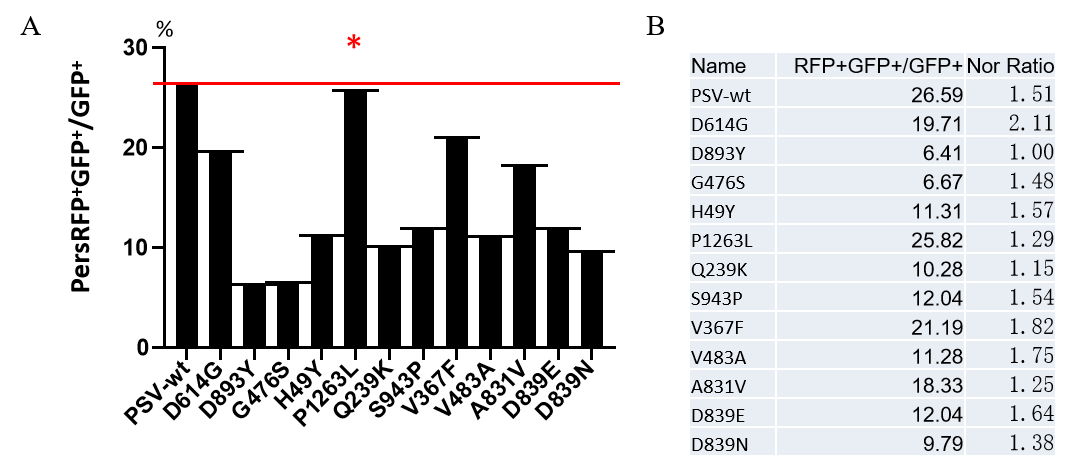
GeneMedi-SARS-CoV-2 WT and Spike Mutation Variants Pseudovirus (PSV) Based Neutralizing Assay with GeneMedi's anti-2019-nCoV Spike Neutralizing antibodies (Nabs)


Reference
1.Lois Zoppi, B.A., What are Neutralizing Antibodies?
2.Morales-Nunez, J.J., et al., Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines (Basel), 2021. 9(12).
3.Niu, L., et al., A Structural Landscape of Neutralizing Antibodies Against SARS-CoV-2 Receptor Binding Domain. Front Immunol, 2021. 12: p. 647934.
4.Bentley, E.M., S.T. Mather and N.J. Temperton, The use of pseudotypes to study viruses, virus sero-epidemiology and vaccination. Vaccine, 2015. 33(26): p. 2955-62.




