Lentivirus vector system (lentivirus expression system, lentivirus packaging plasmid system)
Lentivirus vector system - Introduction
GeneMedi’s lentivirus Vector System, also named the lentivirus expression system or lentivirus packaging plasmid system, is a powerful tool for in-vitro & in-vivo gene delivery, shRNA mediated RNA interference (RNAi), gene editing and stable cellline development. You can easily produce a recombinant lentivirus particle in HEK293T or HEK293FT cell line in high titer using GeneMedi’s lentivirus Vector System.
The Genemedi lentivirus vector system including 3 lentivirus packaging plasmids: the lentivirus expression plasmids with different promoters and tags, an envelope protein VSV-G expressing plasmid pMD2G and a packaging plasmid pSPAX2 expressing Gag-pol and Tat.
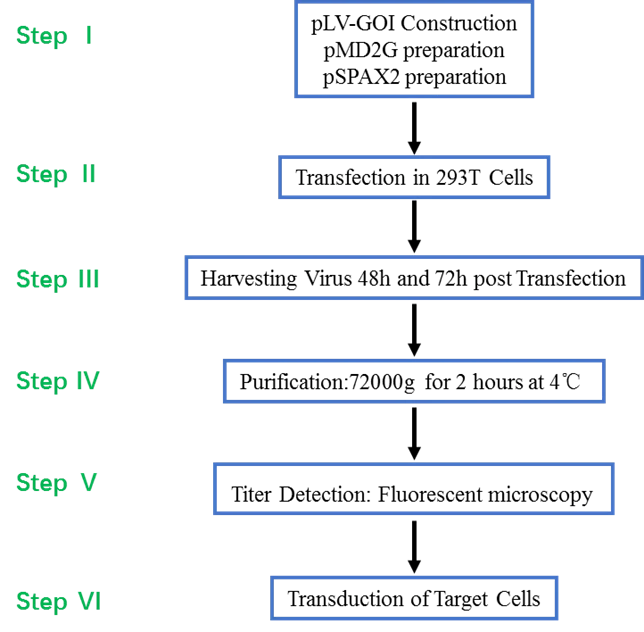
Note:
pLV-GOI Construction
pMD2G preparation
pSPAX2 preparation
Transfection in 293T Cells
Harvesting Virus 48h and 72h post Transfection
Purification:72000g for 2 hours at 4℃
Titer Detection: Fluorescent microscopy
Transduction of Target Cells
GeneMedi has developed a variety of lentivirus expression vectors with different expression cassettes, containing kinds of verified promoters and reporters including GFP, zsgreen, RFP, mcherry and luciferase. The GeneMedi’s lentivirus expression vectors have been proved very suitalble for unique gene overexpression or shRNA-mediated knock-down (also called RNAi (RNA interference ). You can also achieve gene knock-out(KO) or gene editing using our Crispr-cas9-gRNA lentivirus expression vector.
Introduction to Lentivirus Vector System (Lentivurs packaging and expression system)
Lentivirus are based on HIV-1 (human immunodeficiency virus type I), which has been one of the most widely used gene therapy vectors. As a powerful tool for introduction of exogenous genes, there are many advantageous features of lentivirus vectors, such as mediating efficient transfection and long-term expression of exogenous genes in both dividing and non-dividing cells. To date, lentivirus vector has been widespread utilized in various cell lines for gene overexpression, RNA interference, microRNA research and in vivo animal experiments.
A lentivirus packaging system including the lentivirus expression vector, the lentivirus package plasmid and helper plasmid. In order to meet different infection needs, Genemedi has launched a variety of lentivirus packaging system plasmids with different fluorescent labels (such as GFP, RFP, mCherry and so on), and various promoters or tags, which may also allow researchers to produce lentiviruses by themselves as they need.
Lentivirus vector system
Lentivirus expression vectors (lentivirus expression plasmids)
Note: Industry R&D excludes CRO&CDMO&CXO; Others includes CRO&CDMO&CXO&CMC&Manufacturing company
Helper plasmids of lentivirus packaging
PMD2G: an envelope protein VSV-G expressing plasmid
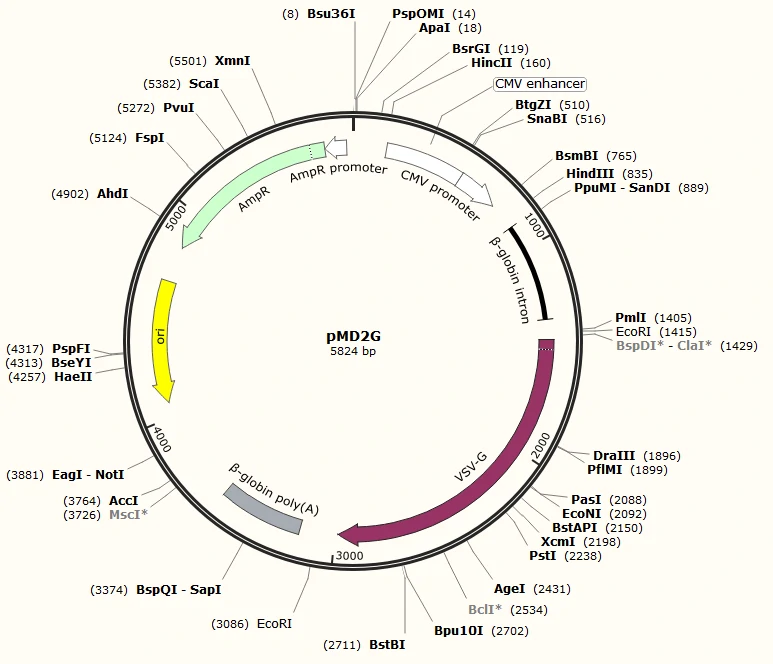
Click here to download SnapGene Viewer
pSPAX2: lentivirus packaging plasmids expressing Gag-pol and Tat
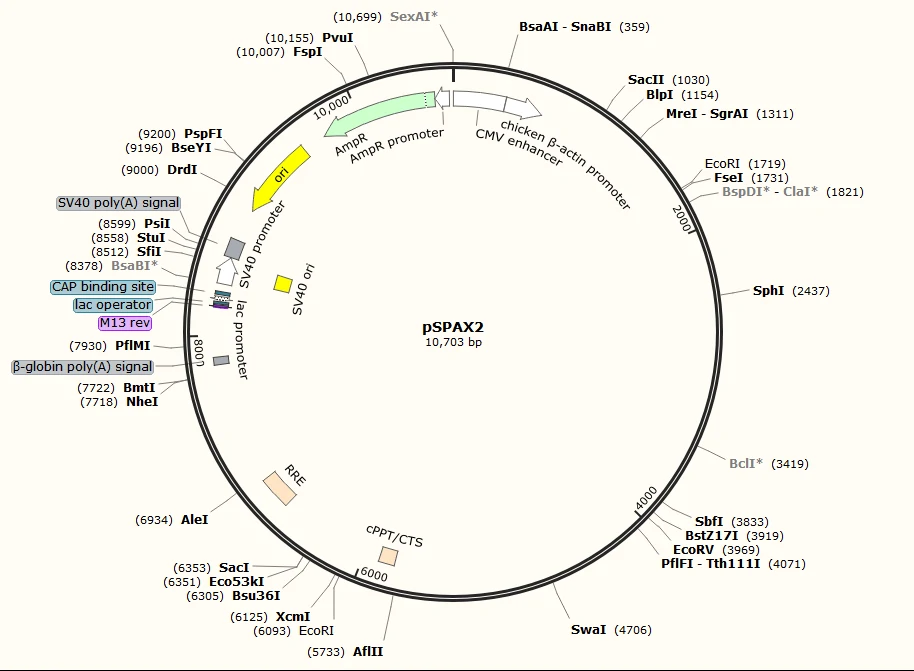
Advantages
1. Customized cloning for any other gene ORF expression, shRNA/miRNA and CRISPR/Cas9. No known immunogenic proteins generated.
2. High titer. 108TU/ml or 109TU/ml lentiviral titer for cell line transfection in medium or large scale.
3. With wide range of host. Mediate efficient transfection in both dividing and non-dividing cells.
4. Integration into host cell genome, mediating long-term and stable expression of exogenous genes.
5. Deliver complex genetic elements, such as intron-containing sequences
6. Easy system for vector manipulation and production
Applications and Figures
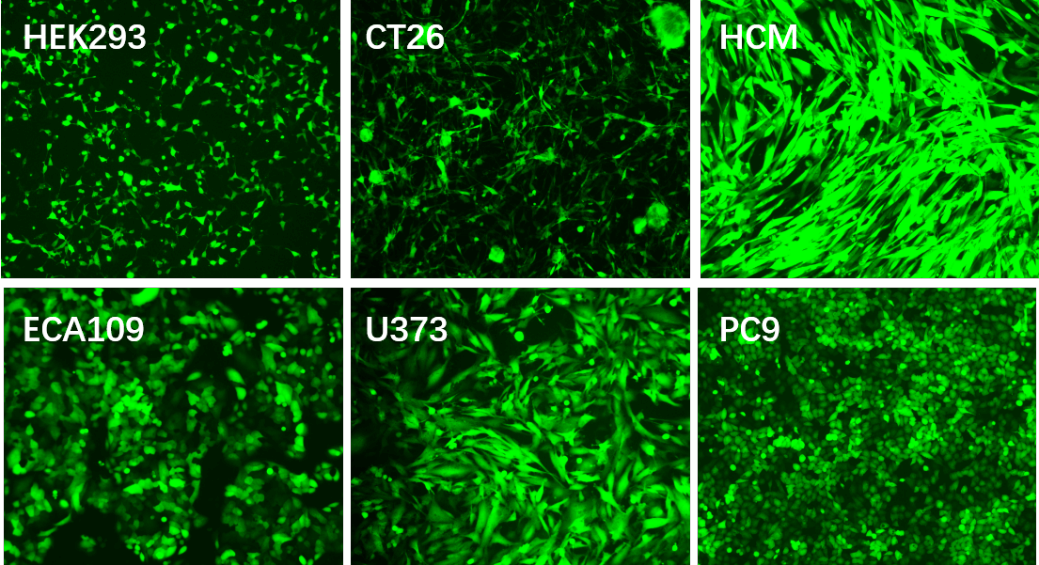
Technical Documents
 Lentivirus User Manual.
Lentivirus User Manual. Reference
1.Wu, J. et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. Journal of Clinical Investigation 125, 4091-4106 (2015).
2.Li, F., Li, S. & Cheng, T. TGF-β1 Promotes Osteosarcoma Cell Migration and Invasion Through the miR- 143-Versican Pathway. Cellular Physiology and Biochemistry 34, 2169-2179 (2014).
3.Liu, Z. et al. miR-451a Inhibited Cell Proliferation and Enhanced Tamoxifen Sensitive in Breast Cancer via Macrophage Migration Inhibitory Factor. BioMed Research International 2015, 207684-207684 (2015).
4.Si, L. et al. Smad4 mediated BMP2 signal is essential for the regulation of GATA4 and Nkx2.5 by affecting the histone H3 acetylation in H9c2 cells. Biochemical and Biophysical Research Communications 450, 81-86 (2014).
5.Han, H., Yang, S., Lin, S. G., Xu, C. S. & Han, Z. Effects and mechanism of downregulation of COX‑2 expression by RNA interference on proliferation and apoptosis of human breast cancer MCF‑7 cells. Molecular Medicine Reports 10, 3092-3098 (2014).
6.Zhang, G., Liu, Z., Cui, G., Wang, X. & Yang, Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumor Biology 35, 11137-11145 (2014).
7.Li, G. et al. CYC1 silencing sensitizes osteosarcoma cells to TRAIL-induced apoptosis. Cellular Physiology and Biochemistry 34, 2070-2080 (2014).
8.Mao, J., Lv, Z. & Zhuang, Y. MicroRNA-23a is involved in tumor necrosis factor-α induced apoptosis in mesenchymal stem cells and myocardial infarction. Experimental and Molecular Pathology 97, 23-30 (2014).
9.Liu, X. et al. Role of human pulmonary fibroblast-derived MCP-1 in cell activation and migration in experimental silicosis. Toxicology and Applied Pharmacology 288, 152-160 (2015).
10.Guan, G. et al. CXCR4-targeted near-infrared imaging allows detection of orthotopic and metastatic human osteosarcoma in a mouse model. Scientific Reports 5, 15244-15244 (2015).
11.Zhang, Y. et al. Role of high-mobility group box 1 in methamphetamine-induced activation and migration of astrocytes. Journal of Neuroinflammation 12, 156-156 (2015).
12.Zhu, T. et al. The Role of MCPIP1 in Ischemia/Reperfusion Injury-Induced HUVEC Migration and Apoptosis. Cellular Physiology and Biochemistry 37, 577-591 (2015).
13.Qian, M. et al. P50-associated COX-2 extragenic RNA (PACER) overexpression promotes proliferation and metastasis of osteosarcoma cells by activating COX-2 gene. Tumor Biology 37, 3879-3886 (2016).
14.Wu, N., Song, Y., Pang, L. & Chen, Z. CRCT1 regulated by microRNA-520 g inhibits proliferation and induces apoptosis in esophageal squamous cell cancer. Tumor Biology 37, 8271-8279 (2016).
15.Wang, Y. et al. Overexpression of Hiwi Inhibits the Growth and Migration of Chronic Myeloid Leukemia Cells. Cell Biochemistry and Biophysics 73, 117-124 (2015).
16.Niu, L. et al. RNF43 Inhibits Cancer Cell Proliferation and Could be a Potential Prognostic Factor for Human Gastric Carcinoma. Cellular Physiology and Biochemistry 36, 1835-1846 (2015).
17.Zhang, H. et al. ZC3H12D attenuated inflammation responses by reducing mRNA stability of proinflammatory genes. Molecular Immunology 67, 206-212 (2015).
18.Deng, X. et al. MiR-146b-5p Promotes Metastasis and Induces Epithelial-Mesenchymal Transition in Thyroid Cancer by Targeting ZNRF3. Cellular Physiology and Biochemistry 35, 71-82 (2015).
19.Zhang, B. et al. HSF1 Relieves Amyloid-β-Induced Cardiomyocytes Apoptosis. Cell Biochemistry and Biophysics 72, 579-587 (2015).
20.Hu, Q. et al. Periostin Mediates TGF-β-Induced Epithelial Mesenchymal Transition in Prostate Cancer Cells. Cellular Physiology and Biochemistry 36, 799-809 (2015).
21.Yang, Z. et al. CD49f Acts as an Inflammation Sensor to Regulate Differentiation, Adhesion, and Migration of Human Mesenchymal Stem Cells. Stem Cells 33, 2798-2810 (2015).
22.Wang, X. et al. MCPIP1 Regulates Alveolar Macrophage Apoptosis and Pulmonary Fibroblast Activation After in vitro Exposure to Silica. Toxicological Sciences 151, 126-138 (2016).
23.Gu, S., Ran, S., Liu, B. & Liang, J. miR-152 induces human dental pulp stem cell senescence by inhibiting SIRT7 expression. FEBS Letters 590, 1123-1131 (2016).
24.Jin, F., Qiao, C., Luan, N. & Li, H. Lentivirus-mediated PHLDA2 overexpression inhibits trophoblast proliferation, migration and invasion, and induces apoptosis. International Journal of Molecular Medicine 37, 949-957 (2016).
25.Liu, Z., Song, Y., Wan, L., Zhang, Y. & Zhou, L. Over-expression of miR-451a can enhance the sensitivity of breast cancer cells to tamoxifen by regulating 14-3-3ζ, estrogen receptor α, and autophagy. Life Sciences 149, 104-113 (2016).
26.Tian, Y. et al. MicroRNA-30a promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting Delta-like 4 expression. Life Sciences 148, 220-228 (2016).
27.Xu, S. et al. MicroRNA-33 promotes the replicative senescence of mouse embryonic fibroblasts by suppressing CDK6. Biochemical and Biophysical Research Communications 473, 1064-1070 (2016).
28.Chen, H., Sun, M., Liu, J., Tong, C. & Meng, T. Silencing of Paternally Expressed Gene 10 Inhibits Trophoblast Proliferation and Invasion. PLOS ONE 10 (2015).
29.Deng, Y. et al. Repair of critical-sized bone defects with anti-miR-31-expressing bone marrow stromal stem cells and poly(glycerol sebacate) scaffolds. European Cells & Materials 27, 13-25 (2014).
30.Zheng, Y. & Xu, Z. MicroRNA-22 induces endothelial progenitor cell senescence by targeting AKT3. Cellular Physiology and Biochemistry 34, 1547-1555 (2014).
31.Yang, X. et al. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumor Biology 36, 383-391 (2015).
32.Wang, W. et al. p53/PUMA expression in human pulmonary fibroblasts mediates cell activation and migration in silicosis. Scientific Reports 5, 16900-16900 (2015).
33.Zhang, S. & Qi, Q. MTSS1 suppresses cell migration and invasion by targeting CTTN in glioblastoma. Journal of Neuro-oncology 121, 425-431 (2015).
34.Wang, P. et al. PFDN1, an indicator for colorectal cancer prognosis, enhances tumor cell proliferation and motility through cytoskeletal reorganization. Medical Oncology 32, 264-264 (2015).
35.Gu, S. et al. Human Dental Pulp Stem Cells via the NF-κB Pathway. Cellular Physiology and Biochemistry 36, 1725-1734 (2015).
36.Huang, G. et al. Clinical and therapeutic significance of sirtuin-4 expression in colorectal cancer. Oncology Reports 35, 2801-2810 (2016).
37.Yan, X., Ye, T., Hu, X., Zhao, P. & Wang, X. 58-F, a flavanone from Ophiopogon japonicus, prevents hepatocyte death by decreasing lysosomal membrane permeability. Scientific Reports 6, 27875 (2016).
38.Ding, W., Tong, Y., Zhang, X., Pan, M. & Chen, S. Study of Arsenic Sulfide in Solid Tumor Cells Reveals Regulation of Nuclear Factors of Activated T-cells by PML and p53. Scientific Reports 6, 19793-19793 (2016).




