Oncolytic virus immunotherapy (HSV, oncolytic adenovirus) and cancer therapy
Cell therapy is a kind of medicine aiming to cure disease or alleviate disease symptoms via direct infusion or transplantation of cells, which can be autologous or allogeneic. With several decades’ development and optimization, immuno-oncology cells (such as T cells, nature killer cells, etc.), stem cells (embryonic stem cells, induced pluripotent stem cells, progenitor cells, etc.) or other genetic re-engineered cells have been widely applied for cell therapy. Numerous cell types have been translated into clinical trials and promising cell therapy outcomes have been achieved from Phase I, Phase II and Phase III trials for a great number of diseases.
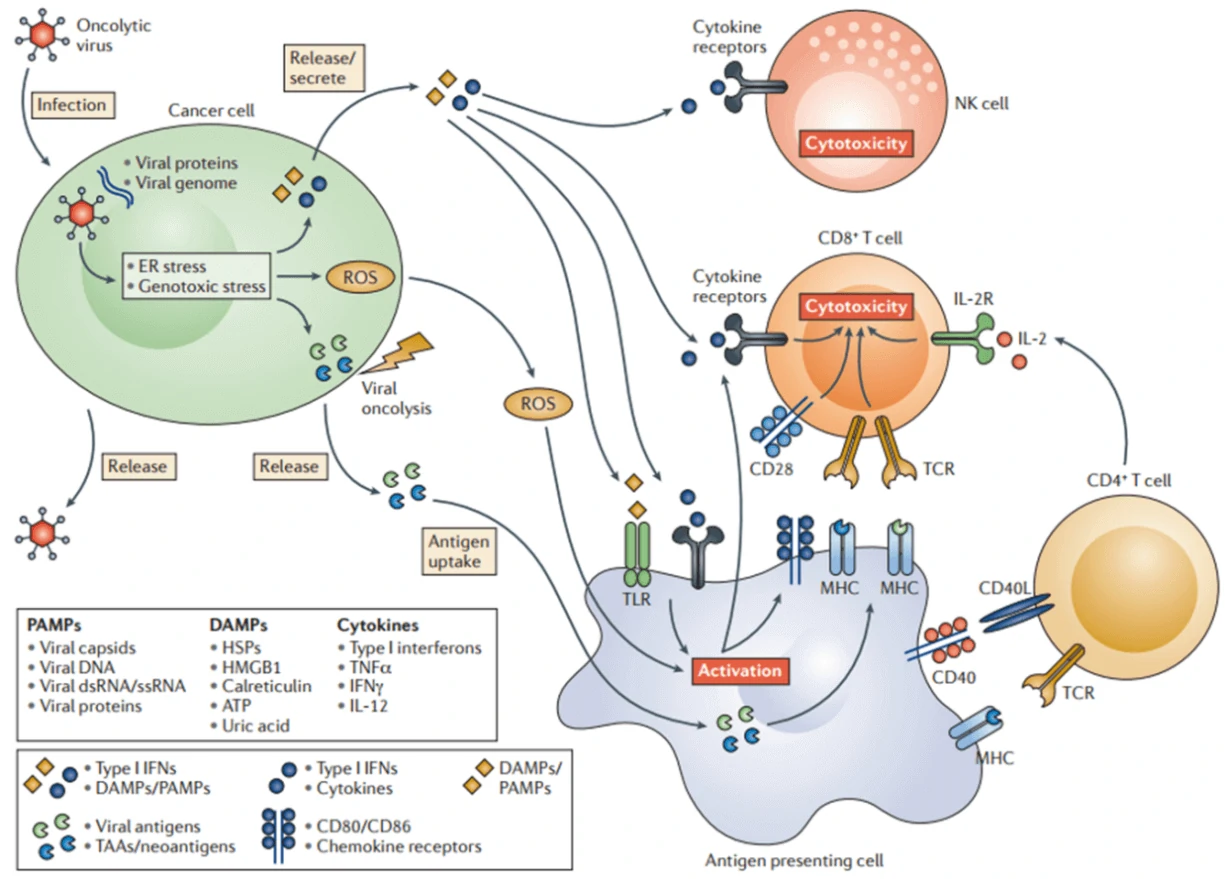
Oncolytic virotherapy is a novel therapeutic modality utilizing oncolytic viruses (such as herpes simplex-, pox-, parvo-, or adenoviruses), which selectively replicate in cancer cells, to directly induce tumor cell lysis or indirectly reactivate human immune system to mediate tumor destruction (Fig. 7) [64]. Through decades’ optimization, a great number of oncolytic virus therapies have been put into clinical trials, such as ONYX-015 (E1B-deleted adenovirus) [65], DNX-2401 (Ad5) [66], CG0070 (Ad5 carrying GM-CSF gene) [67], OBP-301 (Ad5-hTERT-E1A/B) [68], G207 (a conditionally-replicative HSV-1 with ICP34.5 deletion and UL39 disruption) [69], and JX-594 (Pexa-Vec, GM-CSF-enhanced vaccinia virus with TK gene disruption) [67]. Among them, Amgen’s T-VEC (a modified HSV-1 with expression of GM-CSF but deletions in ICP34.5 and ICP47 genes) was the first oncolytic virus approved by America Food and Drug Administration (FDA) for the treatment of melanoma in 2015 [70]. Moreover, when combined with the other therapy, such as Anti-PD-1 immunotherapy, oncolytic virus can significantly promote immune recognition of cancer cell as well as T cell infiltration, and lead to high response rate in patients with advanced melanoma [71].
| Oncolytic viruses | Disease | Description | NCT/Reference |
| Adenovirus | Hepatic carcinoma | Golgi protein 73 (GP73) needed | [72] |
| Adenovirus | Pancreatic carcinoma, prostate cancer | Tat-PTD modified hexon and Ad5/35 | [73] |
| Adenovirus | Gastric cancer | Acetylcholinesterase (AChE) | [74] |
| Adenovirus | Gastric cancer | OBP-301, telomerase-specific | [75] |
| Herpes simplex virus 2 | Colon cancer | No virus modification or co-therapies | [76] |
| Vaccinia | Colon cancer | Viral Thymidine kinase (TK) deficiency | [77] |
| Measles virus | Hepatocellular carcinoma, colon cancer | Retargeted to CD133 | [78] |
| Herpes simplex virus | Ovarian cancer | Interleukin−12 | [79] |
| ONYX-015 (adenovirus) | Malignant mesothelioma cells | EB1 gene deletion | [65] |
| DNX-2401 (adenovirus) | Glioblastoma and gliosarcoma | NCT02197169 | |
| CG0070 (adenovirus) | Bladder cancer | carrying CM-CSF gene | NCT02143804, NCT02365818 |
| OBP-301 (adenovirus) | carrying hTERT-E1A/B | NCT0229385 | |
| HF10 (Herpes simplex virus) | Solid superficial malignant tumors | deletions in neurolatency genes UL43, UL49.5, UL55, and UL56 | NCT02428036 |
| G207 (Herpes simplex virus) | Glioblastoma | a conditionally-replicative HSV-1 with ICP34.5 deletion and UL39 disruption | NCT00157703 |
| JX-594 (Vaccinia Virus) | Hepatocellular Carcinoma, colorectal cancer | GM-CSF-enhanced vaccinia virus with TK gene disruption | NCT02562755 |
| GL-ONC1 (Vaccinia Virus) | Head and neck cancer | TK-inactivated | NCT01443260 |
| Reolysin (Retrovirus) | Metastatic melanoma or pancreatic cancer | NCT02514382, NCT02444546 | |
| Cavatak™ (Coxsackievirus A21) | Melanoma | Overexpress ICAM-1 and DAF | NCT02565992, NCT02316171 |
Taken together, different kinds of immune-oncology cell therapy show great variety in their curative effects. Although promising therapeutic outcomes in acute lymphocytic leukemia (ALL) and large B cell lymphomas have been achieved by CAR-T [80,81], little therapeutic effects were acquired in solid tumors as well as tumors with intracellular antigens, and multiple side effects, such as cytokine release syndrome, prohibited the broad application of CAR-T [82-84]. TCR-T displays better effects for the treatment of hematopoietic cancers and solid tumors, but requires MHC and co-stimulatory or co-inhibitory signals to combat tumors [45]. Without the drawback of CAR-T, CAR-NK is safer than CAR-T and presents excellent tumor elimination ability in both hematopoietic cancers and solid tumors via CAR-dependent and NK receptor-dependent mechanisms [53]. DC-CIK immunotherapy shows improved immunologic function, reduced mortality, and mild adverse effects in both hematologic malignancies and solid tumors, and may be suitable for patients that are intolerance to radiotherapy and chemotherapy [62,85]. Without the need of defined antigens included in the vectors, oncolytic viruses show a durable anti-tumor effects by directly infecting and lysing tumor cells in situ and also activating the host immune response to eliminate tumor cells, thus displaying curative effects on both hematopoietic cancers [86,87] and solid tumors [64]. Moreover, these immune-oncology cell therapies can also be combined with programmed cell death protein 1 (PD-1) antibody or PD-1 inhibitor, showing reduced drug toxicity and enhanced the tumor cytotoxicity [88]. For example, CD19-CAR-T cell carrying the single-chain variable fragment (scFv) of antibody against PD-1 exhibited potent therapeutic effects superior to those of conventional CAR-T cells [89]; oncolytic Herpes Simplex Virus Type 2 encoding an antibody against PD-1 presented an enhanced therapeutic efficacy with a durable antitumor response both in the tumor microenvironment and in the systemic immune system [90].




