Transfection Protocol
Introduction to LipoGene Transfectin Reagent
GeneMedi provide LipoGeneTM transfection reagent which is highly potent cationic lipofection reagent that has been shown to effectively transfect plasmids or siRNA, as well as nucleic acid-protein complexes, into cultured adherent and suspension cell lines. Researchers use LipoGeneTM Reagent for siRNA- and shRNA-based gene knockdown experiments, as well as for gene expression studies.
LipoGene Liposome transfection reagent is highly potent cationic lipofection reagent that has been shown to effectively transfect plasmids or siRNA, as well as nucleic acid-protein complexes, into cultured adherent and suspension cell lines. Researchers use LipoGene Reagent for siRNA- and shRNA-based gene knockdown experiments, as well as for gene expression studies.
Storage: LipoGene can be stored at 4°C. Avoid repeated freezing and thawing. The expiry date is specified on the product label.
Transfection Protocol Procedure
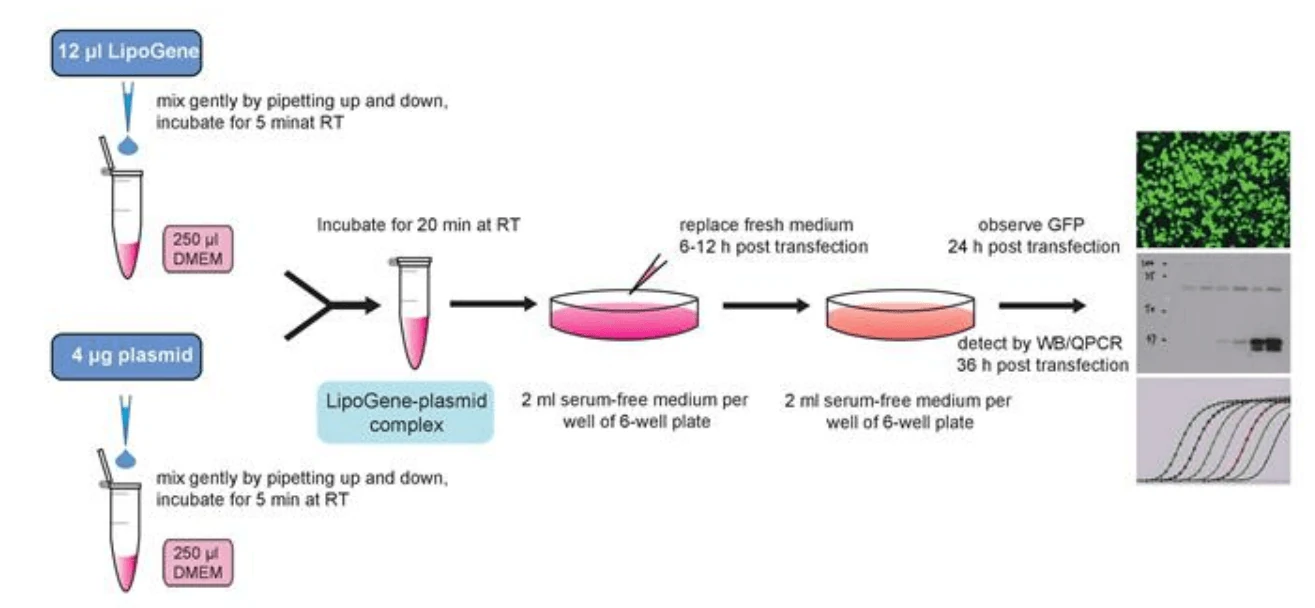
1. Cell culture: Take 293T cells as an example. The day before transfection (20-24 hours) ~0.4×106 cells (the specific cell number depends on the cell size and cell growth rate), were inoculated into a six-well plate, so that they would be 70–90% confluent the next day at the time of transfection.
2. Replace fresh cell culture medium before the following transfection step. The volume of the culture medium is approximately 2 ml.
Note:
Antibiotics, Glutamine, etc. have no ef ect on LipoGene transfection. If LipoGene displayed toxicity to the targetcells, it is recommended to remove the antibiotics from the transfection system.
3. Mix the LipoGene liposome transfection reagent gently.
4. Take a sterile centrifuge tube, add 4 μg plasmid to 250 μl of DMEM solution, and mix gently by pipetting.
Note:
If necessary, DMEM can be replaced by other media such as 1640, MEM, F12, etc., which has no ef ect on the transfection ef iciency of LipoGene. The amounts of plasmids can be appropriately adjusted within the range of 0.1 μg to 4 μg, and the amount of LipoGene is usually 4-8 μl (plasmid volume/LipoGene volume = 1:1 to 1:2). Since the cell type, culture conditions, transfection parameters, etc. will greatly af ect the transfection ef iciency, it is necessary to optimize the plasmid volume/LipoGene volume within the recommended range. For other culture plates or culture vessels, the amount of each reagent can be converted by referring to the Appendix “LipoGene transfection instruction table”.
5. Take another sterile centrifuge tube, add 6 μl LipoGene to 250 μl DMEM solution, mix gently with a pipette, and incubate at room temperature for 5 minutes.
6. Gently mix the DNA solution from steps 4 with LipoGene solution from steps 5 with a pipette.
Note:
Do not vortex or centrifuge.
7. Incubate the LipoGene-DNA mixture for 20 minutes at room temperature. Flocculent deposits may appear, which is a normal phenomenon and will not affect the transfection efficiency.
8. Add 500 μl of LipoGene-DNA mixture to one well of the six-well plate, either in adherent or suspension cells, then shake and mix gently in the shape of “8”.
9. Note: For adherent cells, as described above, simply add the LipoGene-DNA complex to the cells and incubate for 6 hours before changing the solution. If transfected suspension or semi-suspended cells, it is recommended to use a flat-angle centrifugation method, after adding the appropriate amount of LipoGene-DNA complex to the cell culture dish (step 8), seal the sealing film, put it into a flat-angle centrifuge, low speed (200 g) Centrifuge for 1.5 hours, then put it into the incubator for 6 hours and then change the medium.
10. After 6-12 hours incubation, replace the culture medium with a complete medium and continue to culture.
Note:
LipoGene-DNA mixtures are usually suf icient to produce high transfection ef iciency when incubated with cells for six hours. Most cells were cultured with LipoGene-DNA for up to 72 hours without obvious cytotoxicity. However, the replacement of fresh medium 6 hours after transfection can improve the transfection ef iciency of some cells which grow very fast. For some easily transfected cells such as HEK-293T cells, whether change of transfection solution or not depends on cell growth status, and it is not necessary to change the transfection solution to improve the transfection ef iciency.
11. Operations after transfection:
1) For gene expression, transfection efficiency can be detected 24-40 hours later. The expression of GFP or other fluorescent genes can be observed with fluorescence microscopy.
2) For construction of stable cell lines, appropriate screening drugs such as G418 or puromycin can be added 24 hours post transfection to screen for stable cell lines
PRECAUTIONS
1. Use high-purity DNA (A260/A280=1.8) to help achieve higher transfection efficiency. For plasmids, high-quality and endotoxin-free extraction is recommended using the plasmid extraction kit from Qiagen.
2. The state of the cell will greatly affect the efficiency of transfection, so the cells should be in a good state of growth before transfection.
3. This kit contains no DMEM medium, prepare it by yourself. The other media such as 1640, MEM, alpha-MEM, F12, DMEM/F12 and M199 can also be used for transfection experiments.
4. LipoGene™ liposome transfection reagent can’t be vortexed or centrifuged. Just gently shake and mix it when this reagent has been left standing for a long time.
5. Avoid prolonged exposure to air, which may decrease transfection efficiency.
6. For the health and safety considerations, please perform transfection in a cell culture room that meets the cleanliness requirements, and wear a lab coat and disposable gloves, a mask, and a sterile cap.
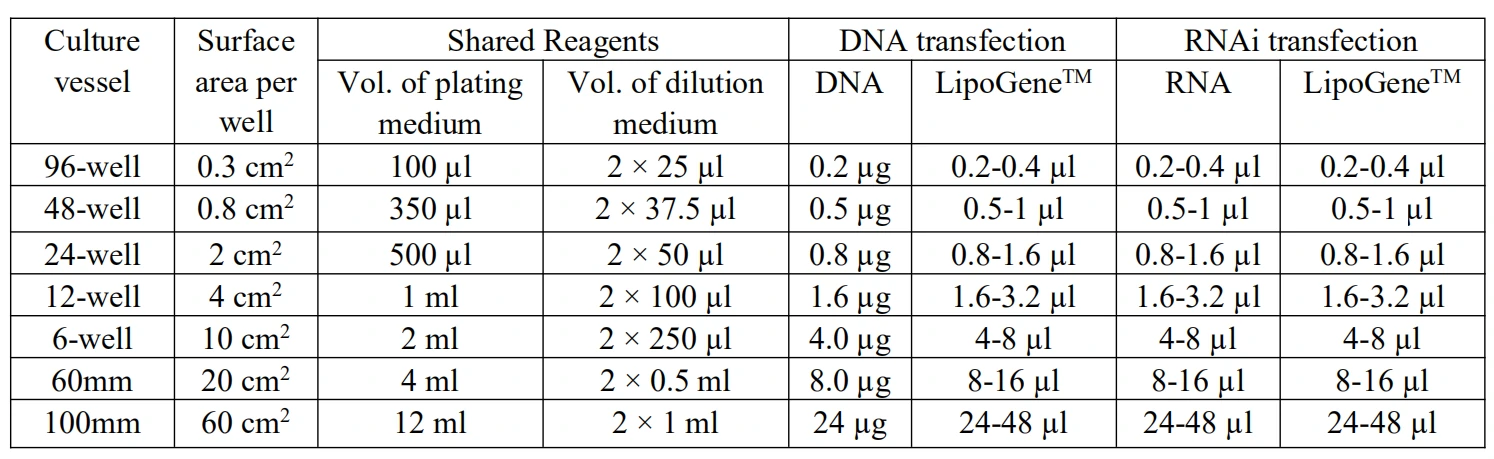
7. LipoGene transfection instruction table: