AAV-LC3 production service for Autophagy Flux Detection
SOCAIL MEDIA

customized AAV production
Customize Option
| Autophagy Flux detection vector | GM-AAVPAU01: AAV-CMV-mRFP-GFP-LC3 GM-AAVPAU02: AAV-CMV-GFP-LC3 |
| AAV Packaging Serotype | AAV1, AAV2, AAV2 variant (Y444F), AAV2 variant (Y272F, Y444F, Y500F, Y730F), AAV2 variant (Y444F, Y730F, Y500F, Y272F, Y704F, Y252F), AAV2 variant (AAV2.7m8), AAV5, AAV6, AAV8, AAV8-1m, AAV8-2m, AAV8 variant (Y733F, Y447F, Y275), AAV9, AAV-Rh.10, AAV-DJ, AAV-DJ/8, AAV-Retro, AAV-PHP.B, AAV-PHP.eB, AAV-PHPS, |
| AAV Vector Production Scale | 2E+11 vg – 1E+15 vg |
| Form | Frozen form |
| Suitable Types of Infection | In vivo infection in animals |
| Sipping and Storage Guidelines | Shipped by dry ice, stored at -80 ℃, effective for 1 year. Avoid repeatedly freezing and thawing. |
Customer Who View This Product Also Viewed
Introduction to AAV-LC3 production service for Autophagy Flux Detection
Premade LC3 Autophagy Biosensors Products and user manual
|
Adeno associated virus(AAV) AAV-GFP-LC3 Autophagy Biosensor AAV-mRFP-GFP-LC3 Autophagy Biosensor |
Adenovirus Adv-GFP-LC3 Autophagy Biosensor Adv-mRFP-GFP-LC3 Autophagy Biosensor |
Lentivirus Lv-GFP-LC3 Autophagy Biosensor Lv-mRFP-GFP-LC3 Autophagy Biosensor |
LC3 Autophagy Biosensors User Manual
Autophagy
Autophagy is a self-degradative process in cell that is important for balancing sources of energy at critical times in development and in response to nutrient stress. For autophagy study, Genemedi supply autophagy biosensor, in which GFP and/or RFP tags are fused at the C-termini of the autophagosome marker LC3, allowing to detect the intensity of autophagy flux in real-time with more accuracy, clarity and intuitiveness. These biosensors provide an enhanced dissection of the maturation of the autophagosome to the autolysosome, which capitalizes on the pH difference between the acidic autolysosome and the neutral autophagosome. The acid-sensitive GFP will be degraded in autolysosome whereas the acid-insensitive RFP will not. Therefore, the change from autophagosome to autolysosome can be visualized by imaging the specific loss of the GFP fluorescence, leaving only red fluorescence.
Adeno associated virus (AAV)
Adeno associated virus (AAV) is one kind of human parvovirus. Recombinant AAV vectors can efficiently transfect various cell types, including dividing and quiescent cells, and induce persistent gene expression in vivo without integrating into host genome and causing any disease. These features make AAV an attractive candidate in the application of gene delivery for gene therapy and human disease model establishment. To date, AAV has been proved as the most excellent gene therapy vector. Over 204 clinical trials have been carried out using AAV vectors for gene delivery, and promising gene therapy outcomes have been achieved from clinical trials for a great number of diseases.
AAV-LC3 production service for Autophagy Flux Detection
Taking advantage of RFP-GFP-LC3 and GFP-LC3 labeling system, Genemedi has launched the production service of AAV-RFP-GFP-LC3 and AAV-GFP-LC3, which can be used to observe autophagy flux and monitor the intensity of autophagy flux in real-time in vivo or in vitro.
Advantages
1. Real time and quantitative in vivo autophagy flux detection.
2. High resolution, more accuracy and sensitivity, than traditional approaches.
3. Safety. The wild type Adeno Associated Virus (AAV) has not currently been known to cause disease, and further security of recombinant AAV is ensured after removal of most AAV genome elements.
4. Low immunogenicity. AAV causes a very mild immune response, lending further support to its apparent lack of pathogenicity.
5. Broad range of host and specificity targeting. AAV has the ability to infect both dividing and quiescent cells, allowing genetic material to be delivered to a highly diverse range of cell types. More than 12 AAV serotypes and a variety of capsid engineered AAV vectors can be selected according to their tissue tropisms.
6. Stable physical properties. AAV is still alive at 60℃ and resistant to chloroform.
Applications and Figures
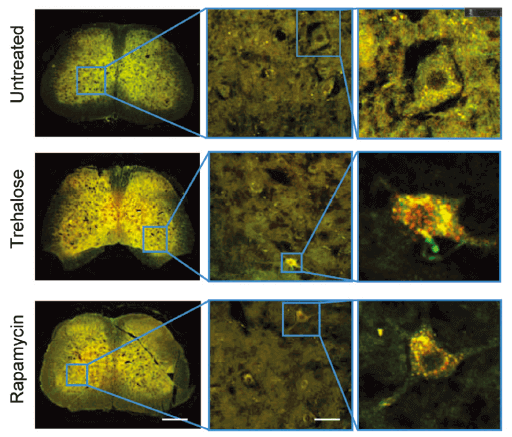
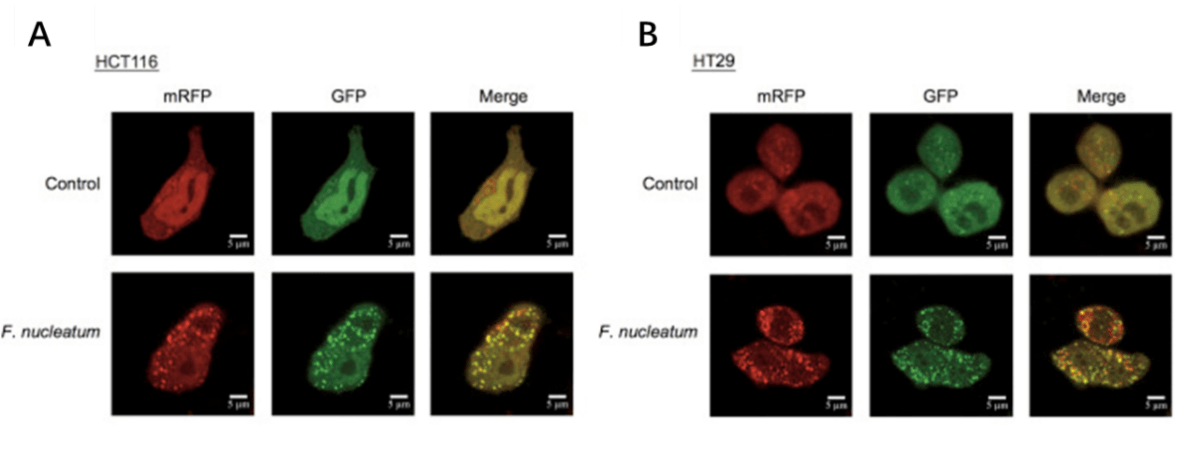
Quality control description
AAV capsid contains VR1 82kDa, VR2 72kDa and VR3 62kDa, which can be detected using the method of polyacrylamide gel electrophoresis (PAGE) followed by silver staining or Coomassie blue staining. Pure AAV should display only three major protein bands, such as shown in Fig 3.
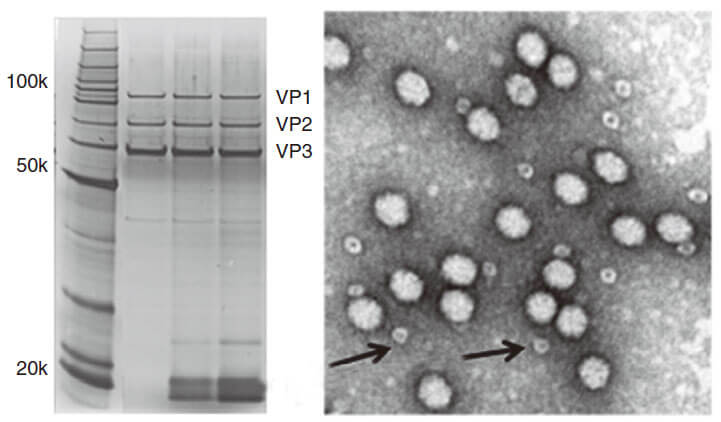
Technical Documents
1. For further information about AAV administration and transduction results, please see the AAV(adeno associated virus) User Manual.
2. For more information about how to use in vivo Autophagy Flux Detection with AAV vectors, please refer to the AAV-LC3 Autophagy Flux Detection Manual.
Reference
1.Yu T, F Guo, Y Yu, T Sun, D Ma, J Han, Y Qian, I Kryczek, D Sun, N Nagarsheth, Y Chen, H Chen, J Hong, W Zou and JY Fang. (2017). Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 170:548-563 e16.
2.Yuan Y, Y Zheng, X Zhang, Y Chen, X Wu, J Wu, Z Shen, L Jiang, L Wang, W Yang, J Luo, Z Qin, W Hu and Z Chen. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13:1754-1766.
3.Shao BZ, P Ke, ZQ Xu, W Wei, MH Cheng, BZ Han, XW Chen, DF Su and C Liu. (2017). Autophagy Plays an Important Role in Anti-inflammatory Mechanisms Stimulated by Alpha7 Nicotinic Acetylcholine Receptor. Front Immunol 8:553.
4.Liu M, K Liang, J Zhen, M Zhou, X Wang, Z Wang, X Wei, Y Zhang, Y Sun, Z Zhou, H Su, C Zhang, N Li, C Gao, J Peng and F Yi. (2017). Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 8:413.
5.Chen ZH, WT Wang, W Huang, K Fang, YM Sun, SR Liu, XQ Luo and YQ Chen. (2017). The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ 24:212-224.
6.Zhang Y, K Shen, Y Bai, X Lv, R Huang, W Zhang, J Chao, LK Nguyen, J Hua, G Gan, G Hu and H Yao. (2016). Mir143-BBC3 cascade reduces microglial survival via interplay between apoptosis and autophagy: Implications for methamphetamine-mediated neurotoxicity. Autophagy 12:1538-59.
7.Luo T, J Fu, A Xu, B Su, Y Ren, N Li, J Zhu, X Zhao, R Dai, J Cao, B Wang, W Qin, J Jiang, J Li, M Wu, G Feng, Y Chen and H Wang. (2016). PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy 12:1355-71.
8.Hua F, K Li, JJ Yu, XX Lv, J Yan, XW Zhang, W Sun, H Lin, S Shang, F Wang, B Cui, R Mu, B Huang, JD Jiang and ZW Hu. (2015). TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat Commun 6:7951.
9.Zhang X, Y Yuan, L Jiang, J Zhang, J Gao, Z Shen, Y Zheng, T Deng, H Yan, W Li, WW Hou, J Lu, Y Shen, H Dai, WW Hu, Z Zhang and Z Chen. (2014). Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 10:1801-13.
10.Li C, W Sun, C Gu, Z Yang, N Quan, J Yang, Z Shi, L Yu and H Ma. (2018). Targeting ALDH2 for Therapeutic Interventions in Chronic Pain-Related Myocardial Ischemic Susceptibility. Theranostics 8:1027-1041.
11.Yuan Y, Y Zheng, X Zhang, Y Chen, X Wu, J Wu, Z Shen, L Jiang, L Wang, W Yang, J Luo, Z Qin, W Hu and Z Chen. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13:1754-1766.
12.Li S, X Dou, H Ning, Q Song, W Wei, X Zhang, C Shen, J Li, C Sun and Z Song. (2017). Sirtuin 3 acts as a negative regulator of autophagy dictating hepatocyte susceptibility to lipotoxicity. Hepatology 66:936-952.
13.Li L, B Li, M Li, C Niu, G Wang, T Li, E Krol, W Jin and JR Speakman. (2017). Brown adipocytes can display a mammary basal myoepithelial cell phenotype in vivo. Mol Metab 6:1198-1211.
14.Feng D, B Wang, L Wang, N Abraham, K Tao, L Huang, W Shi, Y Dong and Y Qu. (2017). Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res 62.
15.Du X, H Hao, Y Yang, S Huang, C Wang, S Gigout, R Ramli, X Li, E Jaworska, I Edwards, J Deuchars, Y Yanagawa, J Qi, B Guan, DB Jaffe, H Zhang and N Gamper. (2017). Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 127:1741-1756.
16.Yang H, J Yang, W Xi, S Hao, B Luo, X He, L Zhu, H Lou, YQ Yu, F Xu, S Duan and H Wang. (2016). Laterodorsal tegmentum





