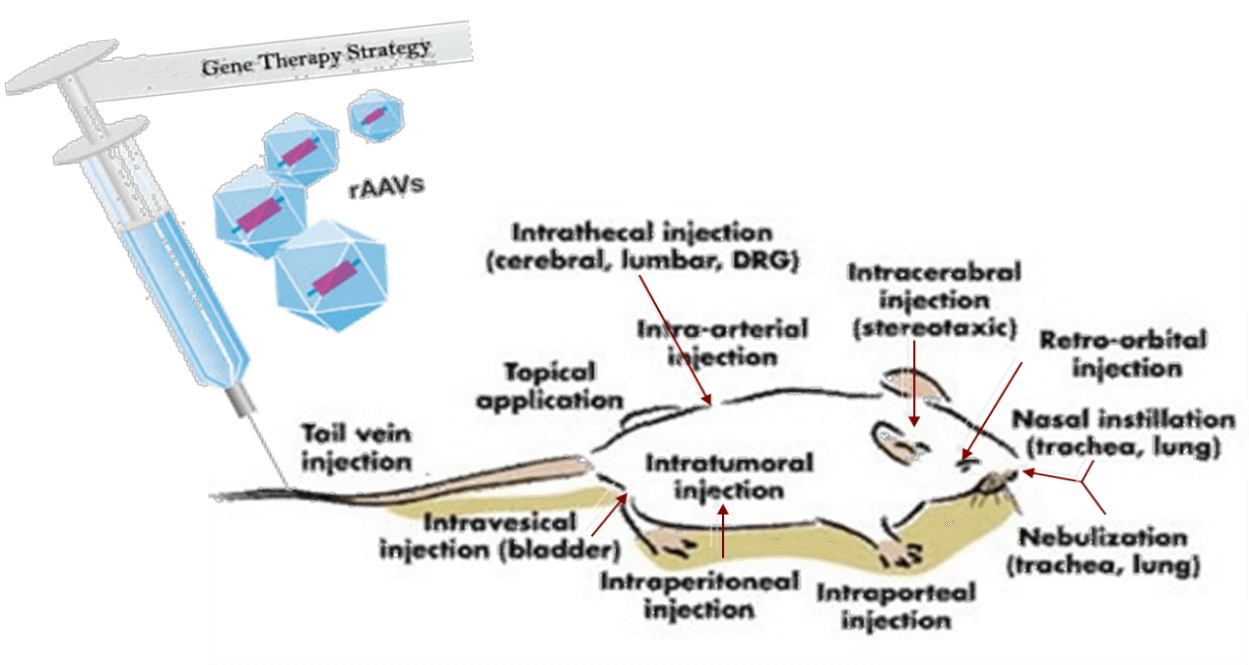
Gene Transfection of Animal Target-organs
DIAGNOSTIC ANTIBODY & ANTIGEN
SERVICES
- Custom AAV Production
- Custom Adenovirus Production
- Custom Lentivirus Production
- Pre-made AAV Production
- Pre-made Adenovirus Production
- Pre-made Lentivirus Production
- AAV-LC3 Autophagy Flux Detection
- Ad-LC3 Autophagy Flux Detection
- Lv-LC3 Autophagy Flux Detection
- AAV Control Virus Production
- CRISPR/Cas9 AAV Production
- CRISPR/Cas9 Adenovirus Production
Gene Transfection, in layman’s words, mainly refers to the increase and decline of gene expression (i.e. gain of function OR loss of function). A classic animal model of gene intervention–genetically engineered mice.Genetically engineered mice included transgenic mice (TG mouse), Knockout mice (KO mouse), and typed mice (KI mouse). In the introduction of CRE-LOXP system, the tissue-specific TG and KO mice were developed.
There is no doubt that genetically engineered mice play a significant role in biomedical research and development, but there are also a number of limiting factors:
1)The genetic intervention that begins with the fertilized egg, because of the long and complex compensation of the developmental process, may have a large deviation from the functional phenotype and the actual phenotype of the gene function.In August 2015, Nature magazine published the Didier Stainier research team found that the fertilized egg knockout egfl7 gene and adult RNA interference block egf17 expression, the pattern of organisms obtained by the phenotype is completely different.
2)Genetically engineered mice have a long cycle and a relatively high cost.For example, researchers need to feed experimental mouse models in animal houses, help mice reproduce, and breed their offspring, etc.
For more information and protocol about Adeno-associated virus, see AAV vector system, AAV production, transduction and AAV gene therapy.
Gene Transfection in animals mediated by viral vectors
At present, the most mainstream virus vector tool has three kinds:Adeno virus, Lentiviral, Adeno-associated virus(AAV).Compared with the limitations of genetically engineered mice, virus carriers have the following advantages:
i)Viral vectors directly carry out acute gene interventions in adult animals, avoiding the problem of developmental decompensation;
ii)Compared with genetically engineered mice, the cycle and maintenance costs of the package are much smaller;
iii)Viral vector-mediated gene intervention can be done after animal modeling, as a gene therapy intervention, closer to the clinical, rather than just completing the study of gene function.
1)Lentivirus vectors: Lentiviral genes are widely used in cell gene transfection to establish a gene-stabilized cell line because of their own genomic integration and long-time expression.But in the animal living (in vivo) level, the lentiviral and adeno-associated viruses are significantly inferior in comparison to the virus—
a.Restriction of lentiviral Titer: Gene transfection in animals has a high demand for the total amount of active viral vectors injected and the volume of injection,Injection volume is often neglected but in fact the most limiting factor, such as the mouse ventricle injection, the volume of injections can not exceed 2ul.The same amount of viral injections, the higher the viral titer, the smaller the volume of injections, and the slow virus due to the titer limit (108~109tu/ml), the amount of virus active virus particles contained in the unit volume is much less than the adenovirus and AAV, so in the animal body (in vivo) gene Transfection application, Lentiviral Transfection is far less effective than adenovirus and AAV.
b. Potential for tumor-causing: lentiviral integration into the genome of host cells forms a stable and theoretically long-time expression. Although the slow virus is generally considered to be relatively safe in practical experiments, this random insertion will theoretically still have the potential risk of inoculating animal-induced tumors.The NIH security level for Lentiviral is rk2+ level, which is the highest risk level in three viruses (adenovirus is RK2 level, AAV is RK1 level).
2)Adenovirus vector: adenovirus vector is characterized by high titer (1011PFU/ML), large insertion fragment, broad spectrum of infection, strong instantaneous expression ability, short time for gene expression.Adenovirus is the first viral vector used in clinical trials of gene therapy, and it is widely used in animal gene transfection.Adenovirus vectors are prominent and distinct in animal gene transfection experiments:
a.Adenovirus can begin to express strongly in the 72 hours of injection intervention, thus having a significant advantage in the acute in vivo gene transfection adenovirus, which is particularly short in the intervention window;
b.The expression of adenovirus vectors in vivo is 2-4 weeks, four weeks after expression of gene expression attenuation is obvious, if the need to extend the expression time, the first injection 2 weeks after the two injections.
c.Adenovirus has a higher immunogenicity, through systemic administration is easier to activate the immune system to produce the corresponding antibodies, affecting the gene transfection effect in vivo, so the adenovirus vector is more of the target organs of the local injection (liver exception). The most common method of adenovirus vector administration is in situ multi-point injection, such as skeletal muscle, myocardium, tumor tissue, etc,generally choose 3-5 injection points, each point around 10ul injection volume.
3)Adeno-associated virus (AAV): Gene transfection artifact in animals
Adeno-associated virus, namely AAV, is the best and safest animal gene transfection tool, compared with other viral vectors such as slow virus, AAV has high titer, very low immunogenicity, long expression time, no pathogenic and pathological toxicity, viral particles of small diffusion range, multi-serum type and has the advantages of relative tissue affinity and so on. AAV has a variety of serotypes, each serum type of target organ affinity is different, which determines that the AAV and other viral vectors are more organ-selective carrier.
| AAV Serotype | Tissue tropism | |||||||
| CNS | Retina | Lung | Liver | Pancreas | Kidney | Heart | Muscle | |
| AAV1 | √ | √ | √ | √ | √ | |||
| AAV2 | √ | √ | √ | |||||
| AAV3 | √ | √ | √ | √ | ||||
| AAV4 | √ | √ | √ | |||||
| AAV5 | √ | √ | √ | √ | ||||
| AAV6 | √ | √ | √ | √ | √ | |||
| AAV7 | √ | √ | ||||||
| AAV8 | √ | √ | √ | √ | ||||
| AAV9 | √ | √ | √ | √ | √ | |||
| AAV-DJ | √ | √ | √ | √ | ||||
| AAV-DJ/8 | √ | √ | √ | |||||
| AAV-Rh10 | √ | √ | √ | √ | √ | |||
| AAV-retro | √ | √ | √ | |||||
| AAV-PHP.B | √ | √ | √ | |||||
| AAV8-PHP.eB | √ | √ | ||||||
| AAV-PHP.S | √ | √ | √ | |||||
AAV is an animal gene transfection artifact, from the heart, liver, brain region, lungs, kidneys, digestive tract, muscle, blood vessels, eyes, inner ear, testicles, scientists can achieve high-efficiency gene transfection of multiple organs.
Multi-organs of multiple serum aav Infection Map
.webp)
Virus and titer: AAV9-GFP, AAV9-pTBG-Luciferase (AAV-Luc), 1.4×1012 vg/ml
Animal: mouse, C57, 2 months
Gene delivery method: tail vein, 100μl
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy, in vivo imaging
Conclusion: intravenous injection of AAV9-pTBG-Luciferase (AAV-Luc) only infects liver cells and no non-specific infection
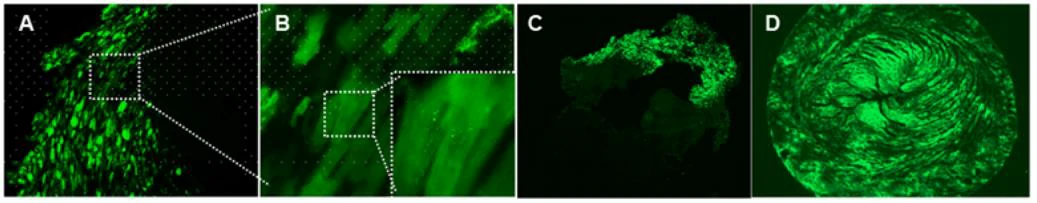
Virus and titer: AAV9-GFP, 1×1012 vg/ml
Animal: Rat, SD, 2 months (Figure 8A, B, C); mouse, C57, 8 months (Figure 8D)
Infection site: heart
Gene delivery method: myocardial in situ injection, 10μl/site, 5 sites in total (Figure 8A, B, C);intraorbital intravenous injection (Figure 8D)
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy
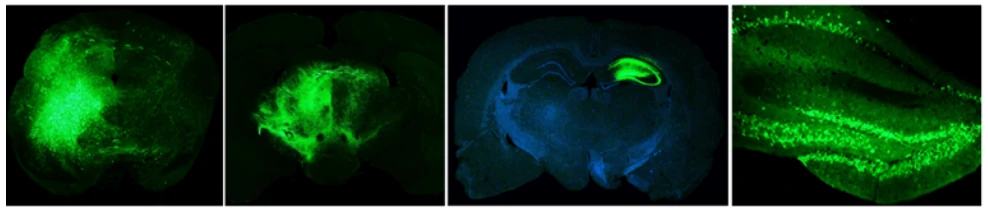
Virus and titer: AAV9-GFP, 1×1012 vg/ml
Animal: Mouse, C57, 2 months
Infection site: Brain
Gene delivery method: Brain localization injection, 1μl
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy
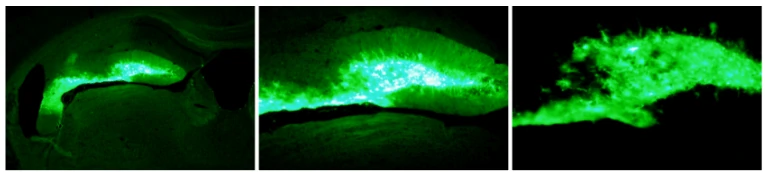
Virus and titer: AAV9-GFAP-GFP, 1×1012 vg/ml
Animal: mouse, C57, 2 months
Infection site: Hippocampus
Gene delivery method: Brain localization injection, 1μl
Determine assay: 3 weeks post infection, wholemount, immunofluorescence microscopy
-and-AAV9-GFP.webp)
Virus and titer: AAV9-Luciferase (AAV-Luc), AAV9-GFP, 1×1012 vg/ml
Animal: Mouse, C57, 2 months
Infection site: Skeletal muscles
Gene delivery method: Muscle in situ injection, 10μl/site, 4 sites in total
Determine assay: 4 weeks post infection, in vivo imaging, frozen section, immunofluorescence microscopy
Virus and titer: AAV-DJ-GFP, AAV9-GFP, 1×1012 vg/ml
Animal: mouse, C57, 2 months
Infection site: Kidney
Gene delivery method: Multiple sites injection in kidney 10μl/site, 6 sites in total
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy
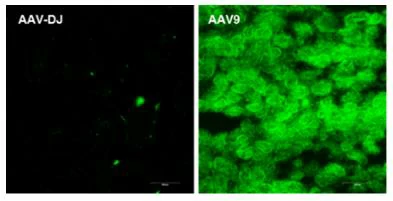
Virus and titer: AAV2 (left) /AAV-DJ (middle) / AAV9 (right), 1×1012 vg/ml
Animal: Nude mouse, 2 months
Infection site: Subcutaneous transplant of bowel cancer cells
Gene delivery method: Tumor injection, 10μl/site, 4 sites in total
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy
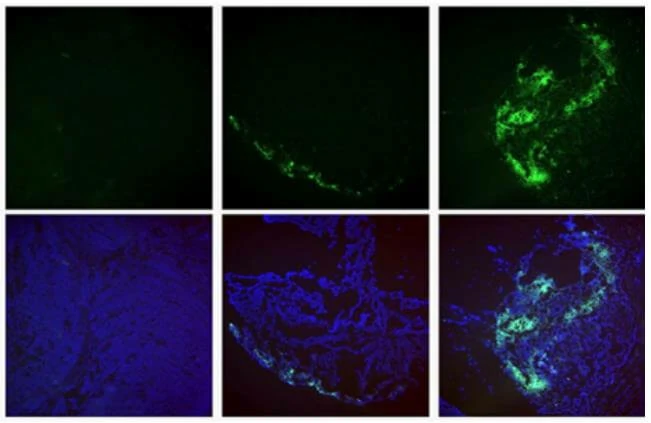
Virus and titer: AAV9-GFP, 1×1012 vg/ml
Animal: mouse, C57, 2 months
Infection site: Mammary fat pad
Gene delivery method: Breast injection, 10μl/site, 4 sites in total
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy

Virus and titer: AAV2 (left) /AAV-DJ (middle) / AAV9 (right), 1×1012 vg/ml
Animal: Nude mouse, 2 months
Infection site: Subcutaneous transplant of bowel cancer cells
Gene delivery method: Tumor injection, 10μl/site, 4 sites in total
Determine assay: 3 weeks post infection, frozen section, immunofluorescence microscopy
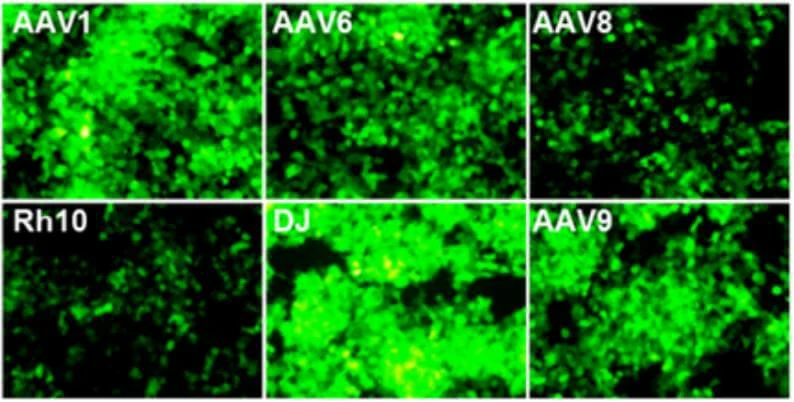
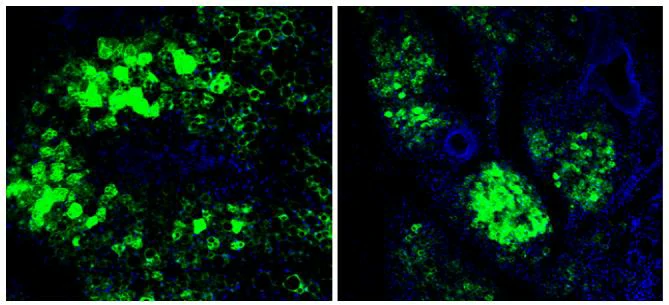
Virus and titer: AAV1/AAV6/AAV8/AAVRh10/AAVDJ/AAV9-GFP, 1×1012 vg/ml
Cells: HEK-293T
MOI: MOI=1×104
Determine assay: 36 hours post infection, immunofluorescence microscopy






