Recombinant Lentivirus System
DIAGNOSTIC ANTIBODY & ANTIGEN
SERVICES
- Custom AAV Production
- Custom Adenovirus Production
- Custom Lentivirus Production
- Pre-made AAV Production
- Pre-made Adenovirus Production
- Pre-made Lentivirus Production
- AAV-LC3 Autophagy Flux Detection
- Ad-LC3 Autophagy Flux Detection
- Lv-LC3 Autophagy Flux Detection
- AAV Control Virus Production
- CRISPR/Cas9 AAV Production
- CRISPR/Cas9 Adenovirus Production
a) Introduction of recombinant lentivirus vector system
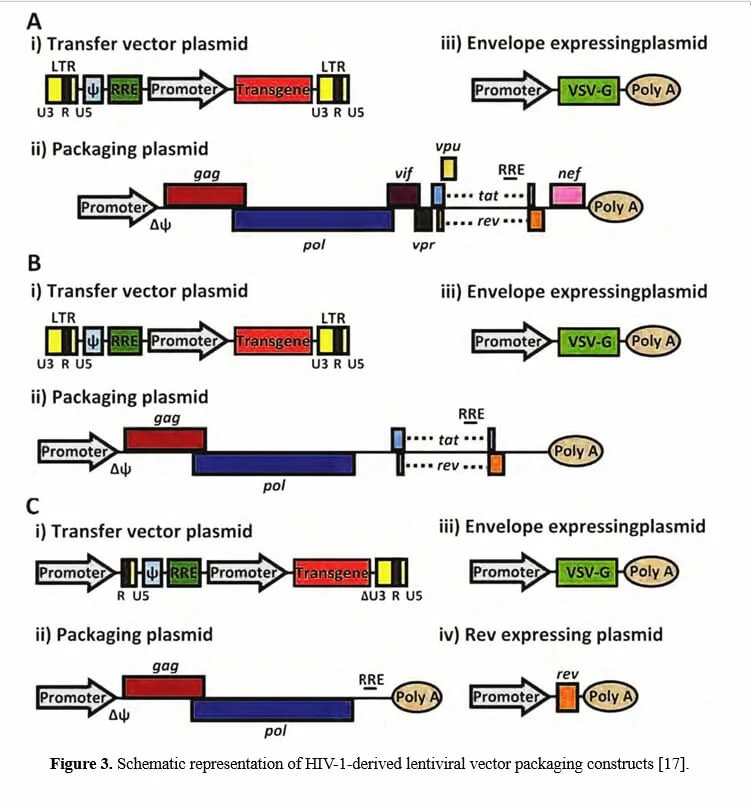
Since wild-type HIV-1 based lentivirus is associated with destruction of host immune system, especially CD4+ helper T lymphocytes, multiple generations of lentivirus vectors have been designed with enhanced safety features and as attractive vectors for gene therapy. To date, there have been three generations of lentivirus vectors (the designing schemes are shown in Figure 3), differing in the extent to which the genome from wild-type HIV-1 is attenuated. The first-generation is three plasmids co-transfection in 293T cells, including all HIV genes with the exception of the env gene in another plasmid, encoding vesicular stomatitis virus G protein (VSV-G) which may improve the stability and broaden the cellular tropism of lentiviral vectors [20,21]. Due to the potential risk for the generation of replication competent lentiviruses (RCL) and of the recombination event during subsequent reverse transcription in transduced cells, researchers have further developed the second-generation lentivirus vectors by eliminating all accessory proteins (vif, vpr, vpu, and nef) via mutation or deletion of these genes from the packaging plasmid, overcoming the safety issue attributable to the first-generation vectors [22-24]. To avoid the homologous recombination between the vector genome and wild-type HIV-1 in case that the lentiviral vectors are used for gene therapy of HIV/AIDS, the third-generation lentivirus vectors have been designed by modifying the 3’ LTR, deleting tat, and providing rev in a separate plasmid, offering the best safety profile in terms of generation of RCL [25-27].
Traditionally, recombinant lentivirus vectors used in Genemedi were prepared with a plasmid containing the transgene flanked by long terminal repeats (LTRs), co-transfected with envelope expressing plasmid pMD2G and packaging plasmid pSPAX2. Once packaged into 293T, recombinant lentiviral vectors will be easily propagated.
b) Recombinant lentivirus system
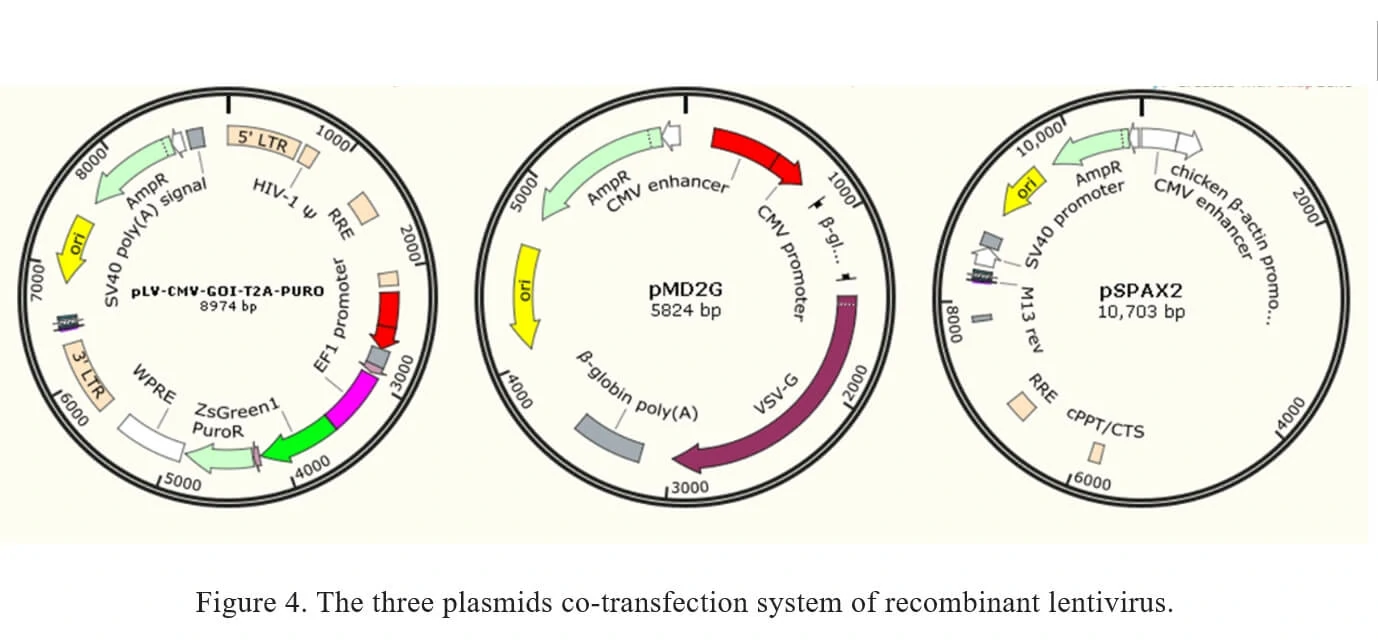
The current method of the recombinant lentivirus production in Genemedi is based on three plasmids co-transfection system. It involves the co-transfection of 3 plasmids into 293T cells as shown in Figure 4.
1. pLV-CMV-MCS-T2A-PURO: a lentivirus LTR-containing plasmid carrying multiple clone sites, which can be cloned into a transgene.
2. pMD2G: envelope expressing plasmid.
3. pSPAX2: packaging plasmid.