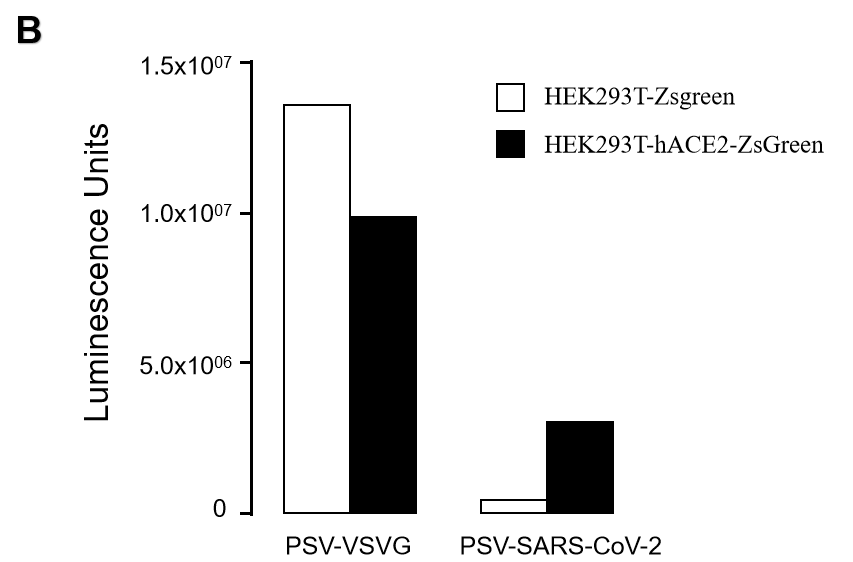Potency Validated COVID-19 SARS-CoV-2 neutralizing antibody, Anti-2019-nCoV Spike (Spike RBD domain) monoclonal neutralizing antibody (human IgG1, human IgM, human IgA, mouse IgG1 and Cynomolgus (Non human primate, NHP) IgG1)
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

Coronavirus disease 2019 (COVID-19) pandemic is caused by SARS-CoV-2 (SARS2, 2019-nCoV) infection, a newly emerged novel coronavirus spreading worldwide. Current efforts are focusing on development of specific antiviral drugs. Therapeutic neutralizing antibodies (NAbs) against SARS-CoV-2(SARS2, 2019-nCoV) will be greatly important therapeutic agents for the treatment of COVID-19. The availability of therapeutic NAbs against SARS-CoV-2 will offer benefits for the control of the current pandemic and the possible re-emergence of the virus in the future, and their development therefore remains a high priority.
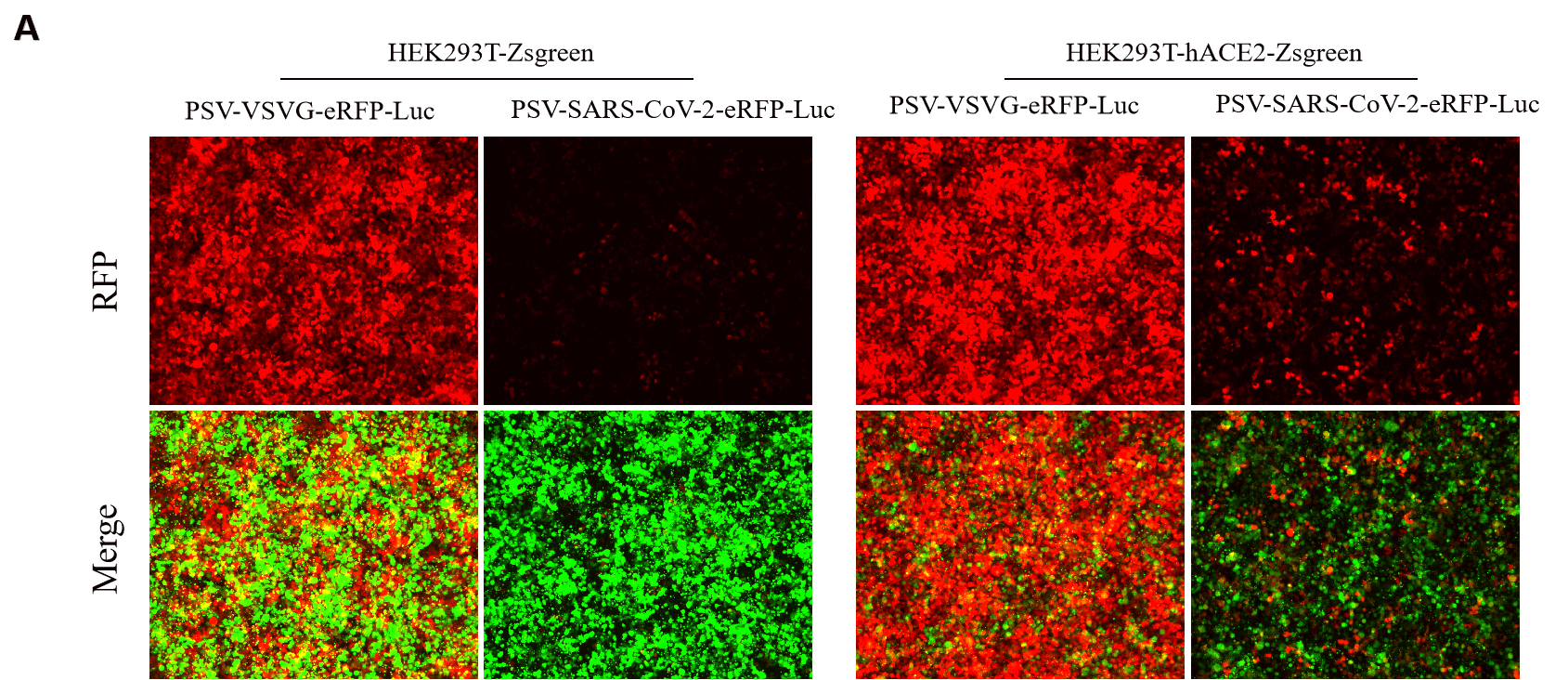
GeneMedi’s NAbs has been validated to reduce SARS-CoV-2 lentivirus-based pseudo virus infectivity and thereby blocking the entry of the Coronavirus to its effector/targeting cell: human ACE2-HEK293T cell.
GeneMedi’s NAbs has been validated by
1. COVID-19 Spike protein and Spike-RBD protein binding affnity. Click here to Spike-RBD(GMP-V-2019nCoV-SRBD001)
2. 2019nCoV pseudotyped virus (PSV) based neutralization assay in 293T-ACE2 effector cell. Click here to SARS-CoV-2 Pseudotyped virus
3. Competitively blocking the binding of ACE-2 receptor with SARS-CoV-2 Spike protein. Click here to ACE2-Fc(GMP-H-ACE2002) 、 SRBD001、 S1S2001
GeneMedi’s Coronavirus neutralizing antibodies (Nabs) can either act as positive control in COVID-19 related vaccines and neutralizing antibodies discovery and development, or act as a benchmark in SARS-CoV-2 neutralization potency assay.
GeneMedi’s validated COVID-19 neutralizing antibodies group including:
GMP-V-2019nCoV-SnAb001(human IgG1),
GMP-V-2019nCoV-SnAb002(human IgM),
GMP-V-2019nCoV-SnAb003(human IGA),
GMP-V-2019nCoV-SnAb004(mouse IgG1),
GMP-V-2019nCoV-SnAb005(Cynomolgus (Non human primate, NHP) IgG1),
GMP-V-2019nCoV-SnAb006(human IgG1),
GMP-V-2019nCoV-SnAb007(human IgG1),
GMP-V-2019nCoV-SnAb008(human IgG1).
Potency Validated COVID-19 SARS-CoV-2 neutralizing antibody
SARS-CoV-2 neutralizing antibody validated post download
–Nab discovery and vaccines evaluation through SARS-CoV-2 wildtype/mutant variants pseudovirus based neutralizing assay(PBNA) and Spike-ACE2 competition binding assay
GeneMedi-SARS-CoV-2 WT and Spike Mutation Variants Pseudovirus (PSV) Based Cell Entry
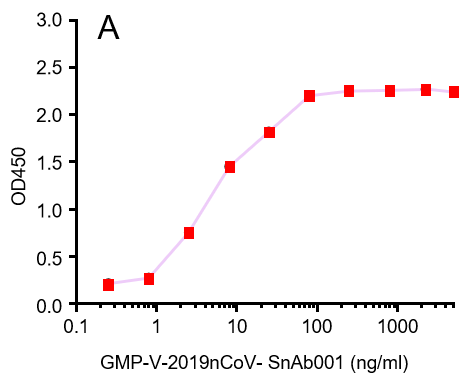
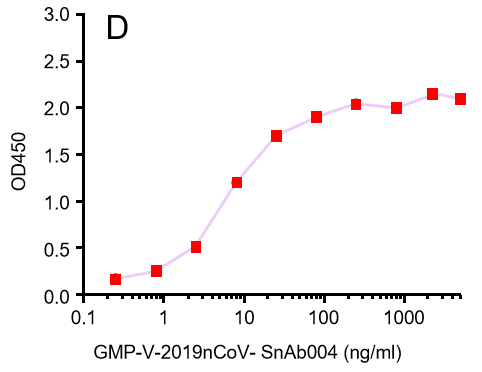
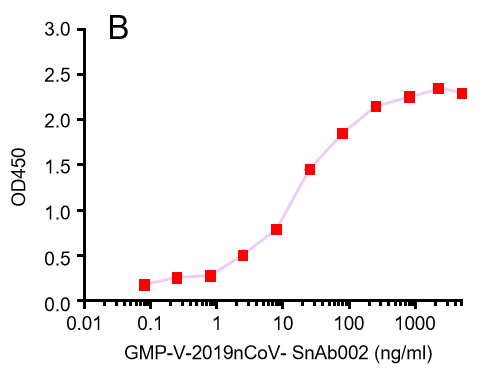
GeneMedi's anti-2019-nCoV Spike Neutralizing antibodies (Nabs) and Spike RBD protein binding validation
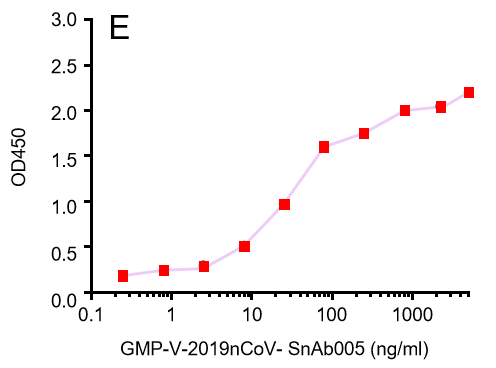
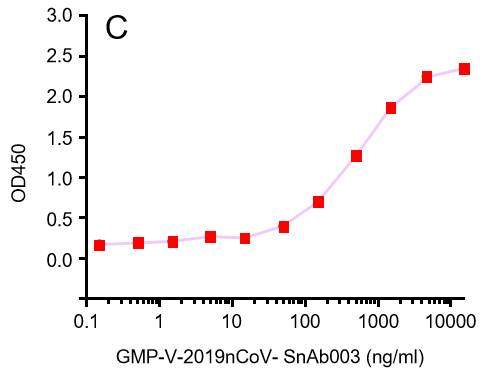
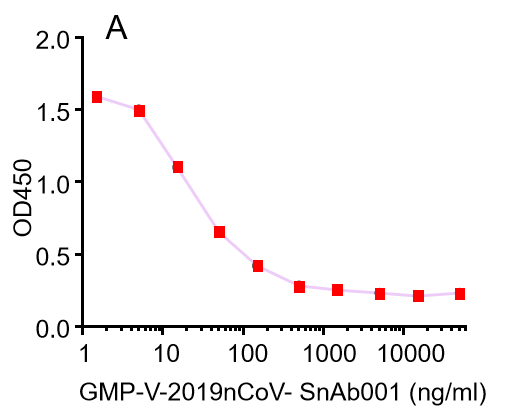
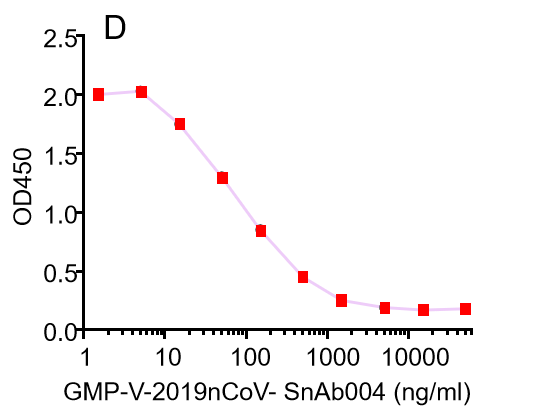
| Cat No. | Product | EC50 (ng/ml) |
| GMP-V-2019nCoV-SnAb001 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1) | 5 |
| GMP-V-2019nCoV-SnAb002 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM) | 18 |
| GMP-V-2019nCoV-SnAb003 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA) | 410 |
| GMP-V-2019nCoV-SnAb004 | Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1) | 6.8 |
| GMP-V-2019nCoV-SnAb005 | Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1) | 28 |
A.GMP-V-2019nCoV-SnAb001:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1)
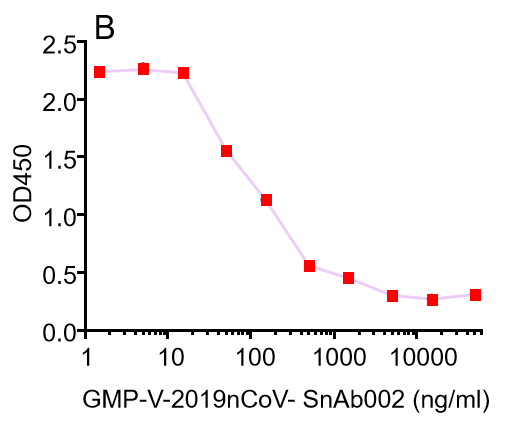
B.GMP-V-2019nCoV-SnAb002:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM)
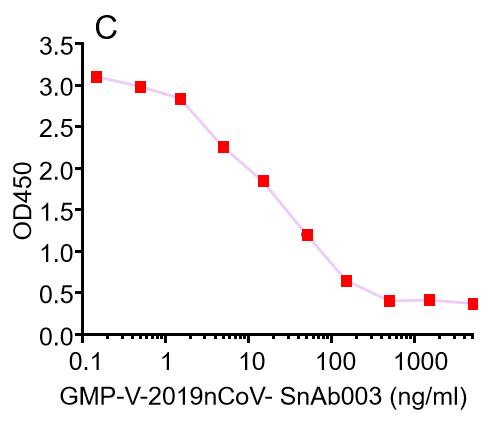
C.GMP-V-2019nCoV-SnAb003:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA)
D.GMP-V-2019nCoV-SnAb004:Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1)
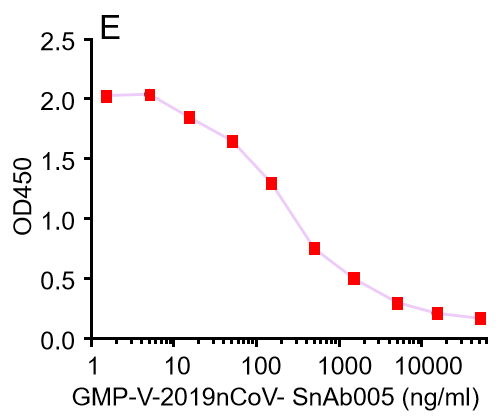
E.GMP-V-2019nCoV-SnAb005:Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1)
GeneMedi's anti-2019-nCoV Spike Neutralizing antibodies (Nabs) competitive binding assay validation
| Cat No. | Product | IC50 (ng/ml) |
| GMP-V-2019nCoV-SnAb001 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1) | 26.3 |
| GMP-V-2019nCoV-SnAb002 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM) | 84.2 |
| GMP-V-2019nCoV-SnAb003 | Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA) | 20.5 |
| GMP-V-2019nCoV-SnAb004 | Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1) | 81.9 |
| GMP-V-2019nCoV-SnAb005 | Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1) | 243 |
A.GMP-V-2019nCoV-SnAb001:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgG1)
B.GMP-V-2019nCoV-SnAb002:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgM)
C.GMP-V-2019nCoV-SnAb003:Anti-2019-nCoV Spike (Spike RBD domain) human monoclonal neutralizing antibody (IgA)
D.GMP-V-2019nCoV-SnAb004:Anti-2019-nCoV Spike (Spike RBD domain) mouse monoclonal neutralizing antibody (IgG1)
E.GMP-V-2019nCoV-SnAb005:Anti-2019-nCoV Spike (Spike RBD domain) Cynomolgus monoclonal neutralizing antibody (IgG1)
GeneMedi-SARS-CoV-2 WT and Spike Mutation Variants Pseudovirus (PSV) Based Neutralizing Assay with GeneMedi's anti-2019-nCoV Spike Neutralizing antibodies (Nabs)


Pseudotyped virus of SARS-CoV-2 Spike Mutation Variants and Effector cells
The outbreak of COVID-19, caused by SARS-CoV-2 (2019-nCoV), has been a global public health threat and caught the worldwide concern. GeneMedi has developed SARS-CoV-2 wildtype and mutation variants pseudovirus production system, from which the SARS-CoV-2 wildtype and mutation variants pseudotyped virus can be handled in biosafety level 2 (BSL-2).
GeneMedi develop COVID-19 related SARS-CoV-2 (2019-nCoV) Pseudotyped virus of SARS-CoV-2 Spike Mutation Variants including D614G, N501Y, E484K, E484Q, L452R, K417N, K417T, S477N, S477G, D253G, and so on.
GeneMedi’s Pseudovirus Based Neutralization Assay (PBNA) is a conventional assay method that is suitable for High-Throughout Screening (HTS) without live virus engaged. The Pseudovirus Based Neutralization Assay can be used for evaluating:
1) Neutralizing antibodies
2) Peptides blockers (peptide inhibitors)
3) Types of Vaccines3
4) Compounds targeting Spike induced cell-fusion.
GeneMedi offers:
1.SARS-CoV-2 Pseudotyped virus packaging and production
2.Effector cells: human ACE2 overexpression stable HEK293T cell lines
3.SARS-CoV-2(2019nCoV) Pseudotyped Virus Based Neutralization Assay service.
Protocol of SARS-CoV-2 Pseudovirus (PSV)-Based Neutralization Assay For Vaccines, therapeutic antibodies, peptides and compounds against COVID-19
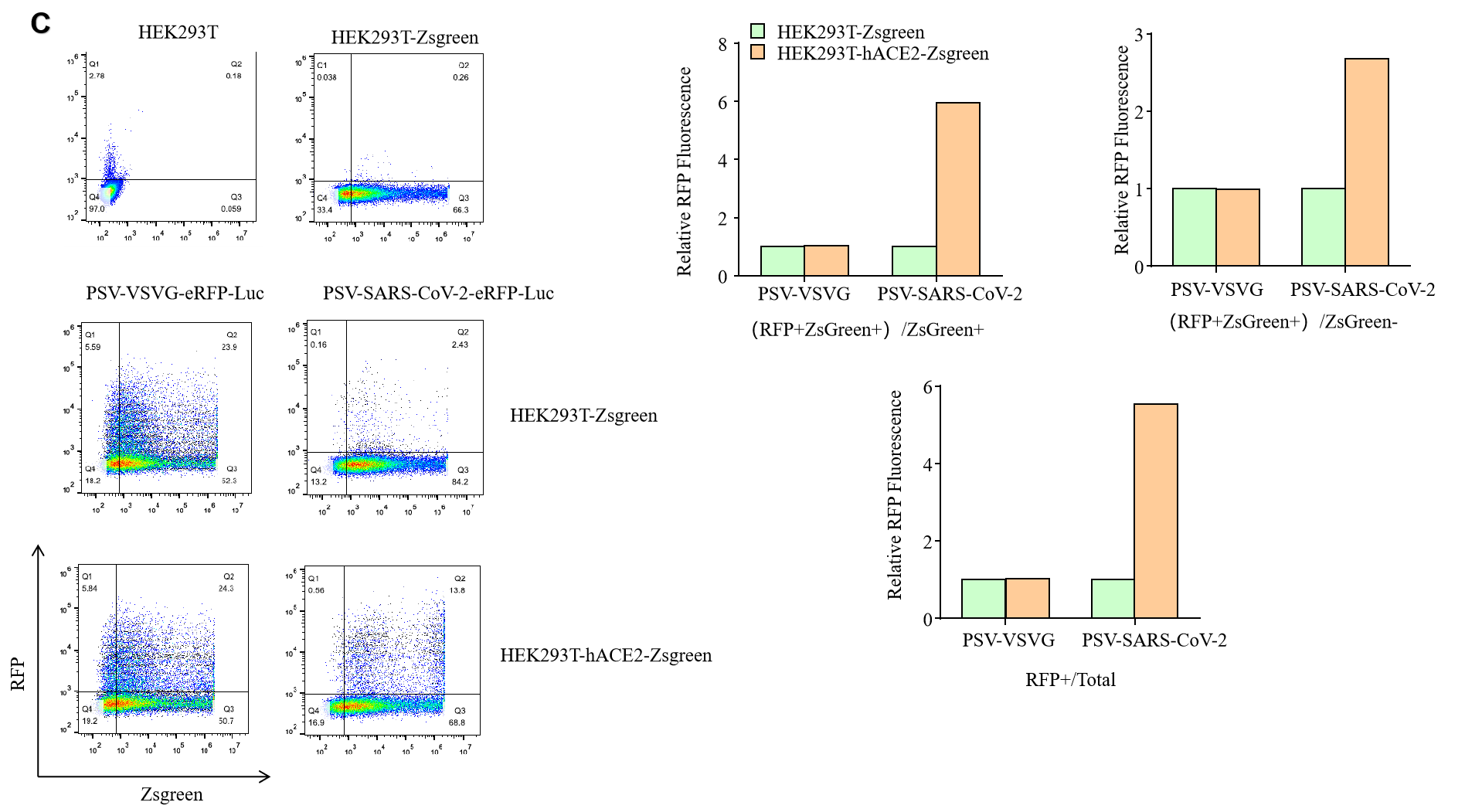
SARS-CoV-2 (2019nCoV) pseudotype virus (pseudovirus, PSV) for COVID-19 related vaccines and neutralizing antibodies evaluation.
The outbreak of COVID-19, caused by SARS-CoV-2 (2019-nCoV), has been a global public health threat and caught the worldwide concern. Due to its high pathogenicity and infectivity1, live SARS-CoV-2 should be handled under biosafety level 3 (BSL-3) conditions. GeneMedi has developed SARS-CoV-2 pseudovirus production system, from which the SARS-CoV-2 pseudotyped virus can be handled in biosafety level 2 (BSL-2)2.
GeneMedi’s SARS-CoV-2 (2019nCoV) pseudotype virus (pseudovirus, PSV) based neutralization assay is a standard evaluation procedure for COVID-19 related vaccines and neutralizing antibodies potency evaluation. GeneMedi’s SARS-CoV-2 PSV is the core ingredient of diagnostics for neutralization serology after vaccinotherapy.
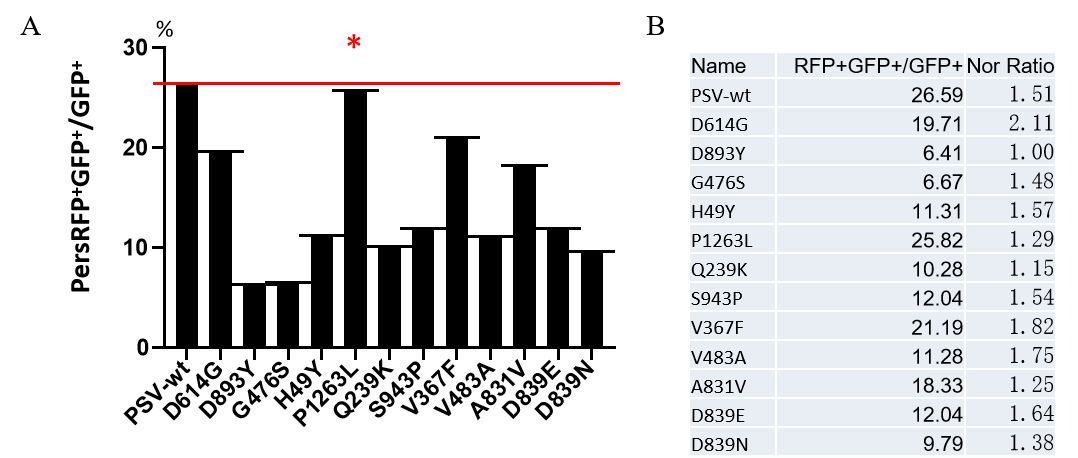
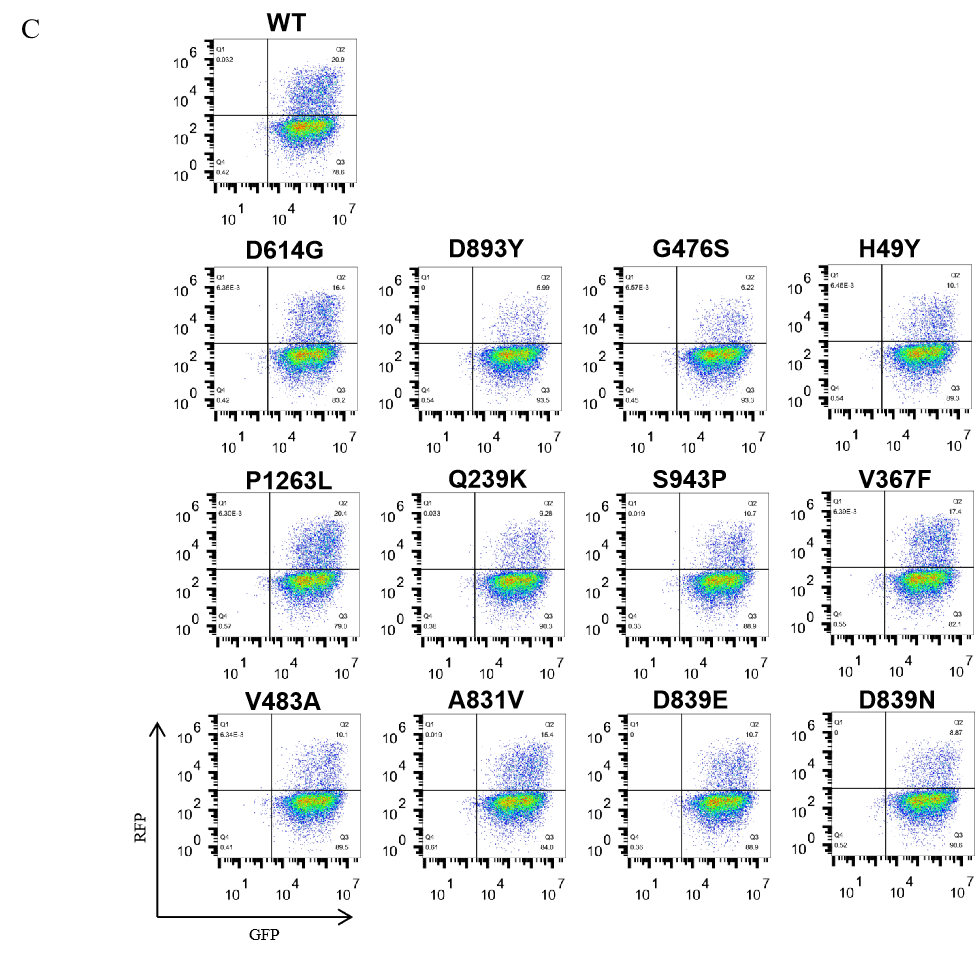
GeneMedi’s SARS-CoV-2 pseudotyped virus includes wildtype and the spike mutation variants (D614G, S943P, V367F, G476S, V483A, H49Y, Q239K, A831V, P1263L, D839Y/N/E: D839Y, D839N, D839E). The GeneMedi’s SARS-CoV2 PSV panel help for all-in-one vaccinotherapy evaluation.
Application-SARS-CoV-2(2019nCoV) Pseudotyped Virus Based Neutralization Assay3
Protocol of SARS-CoV-2 Pseudovirus (PSV)-Based Neutralization Assay
| 100ul PSV-Sample mixture | Volume |
| GM-2019nCoV-PSV01* | 50ul or 5ul |
| Sample(NAbs, peptides, serum, etc) | flexible (According to your own products) |
| Total | add culture medium to 100ul |
| * For GM-2019nCoV-PSV01-1, add 50ul in recommendation (range from20ul~100ul). For GM-2019nCoV-PSV01-2, add 5ul in recommendation. (range from2ul~10ul). | |
Incubate PSV-Sample mixture for 1h at room temperature.
4.Remove the medium of efforter cell in 96-well, add 100ul PSV-Sample mixture to 96well cells for infection. 3wells for a group.
5.Fluorescence imaging (RFP) 72hrs after SARS-CoV-2 PSV infection. The firefly luciferase reporter is measured by following the Promega Luciferase Assay Reagent manual.
Tips
If your samples are serum
A standard curve should be generated using serially diluted Nabs (neutralizing antibodies) as a positive control.
If your samples are therapeutic antibodies or peptides candidates
Dilute the samples into concentration gradient for IC50 value evaluation.
GeneMedi SARS-CoV-2 Pseudovirus (PSV) Based Cell Entry