Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

FDA approved Antibody-drug conjugate (ADC) for clinical use
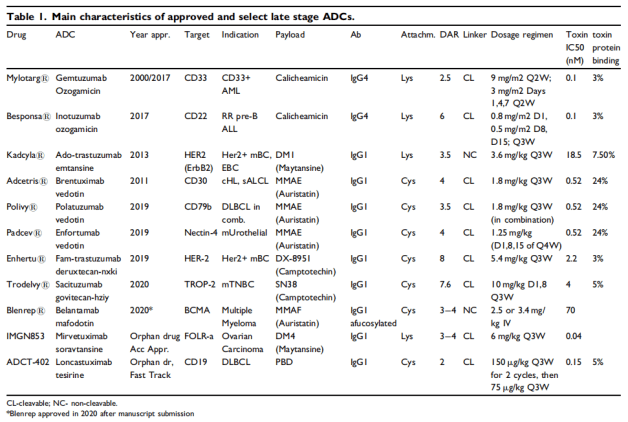
Clinically, most of the ADC drugs approved by FDA are IgG1, and the targets are CD33, CD22, HER2 and so on. The most common payload is MMAE, also contains calicheamicin, DM1. ADC drugs are mainly used in the field of antitumor, which is one of the hot research directions in recent years. At present, 11 ADC drugs have been approved in the world, including Mylotarg (Pfizer), adcetris (Seattle genetics / Takeda), kadcyla (Roche), besponsa (Pfizer), lumoxiti (AstraZeneca), Polivy (Roche), padcev (Seattle genetics / anstelai / MSD), enhertu (AstraZeneca / first third party), trodelvy (immunomedicine), blenrep (GSK) Akalux (Rakuten Aspyrian)1. From the perspective of listed drug R & D enterprises, Pfizer, Seattle genetics, Roche and AstraZeneca have two models respectively, and the other three companies have one model respectively.
Antibody-drug conjugate (ADC) currently under clinical investigation
There are currently 82 novel ADCs in 150 active clinical trials registered with clinicaltrials.gov for cancer patients. Most of the ADCs are currently under investigation in phase 1 trials, while a small percentage has advanced to phase 3. Of the 150 ongoing trials, more than 80% are evaluating ADC safety and efficacy in solid tumors whereas less than 20% are trials for hematological malignancies. There are 43 disclosed targets organized here by the number of ADCs designed to recognize them. Most of these targets are under evaluation by a single ADC, while some are being investigated by several different ADCs. Of the 82 novel ADCs, followed by DNA-damaging molecules, topoisomerase I inhibitors, and finally unique payloads such as TLR agonists, a BCL2-xL inhibitor, and an RNA polymerase II inhibitor. Many payloads can’t be disclosed. Most ADCs under clinical investigation either utilize the conventional cysteine conjugation strategy or site-specific conjugation linker while few conjugate to surface lysines. 2
Novel Antibody-drug conjugate (ADC) in Clinical Trials
More than 80 ADCs are currently in active clinical trials, with a majority in phase I and I/II. Over 80% of the clinical trials are investigating ADC safety and efficacy in various solid tumors, while the remaining trials involve hematological malignancies. HER2 is currently one of the most attractive targets for ADC development, with three anti-HER2 ADCs currently in phase III trials. One such anti-HER2 ADC is RC48, produced by RemeGen, joining an IgG1 anti-HER2 antibody, hertuzumab, to approximately four MMAE molecules via a protease-cleavable valine-citrulline linker through cysteine conjugation.3
Reference
1. Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, Tice DA, Soria JC. Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin Cancer Res. 2019 Sep 15;25(18):5441-5448. doi: 10.1158/1078-0432.CCR-19-0272. Epub 2019 Apr 12. PMID: 30979742.
2. Abedi M , Cohan R A , Mahboudi F , et al. MALDI-MS: a Rapid and Reliable Method for Drug-to-Antibody Ratio Determination of Antibody-Drug Conjugates[J]. Iranian biomedical journal, 2019, 23(6).
3. Xuejing, Yao, Jing, et al. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity[J]. Breast Cancer Research Treatment, 2015.
Product List
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Anetumab biosimilar, Whole mAb ADC, Anti-MSLN Antibody: Anti-MPF/SMRP therapeutic antibody |
Anetumab |
NA |
MSLN |
Whole mAb ADC |
||
Pre-Made Aprutumab biosimilar, Whole mAb ADC, Anti-FGFR2 Antibody: Anti-BBDS/BEK/BFR-1/CD332/CEK3/CFD1/ECT1/JWS/K-SAM/KGFR/TK14/TK25 therapeutic antibody |
Aprutumab |
NA |
FGFR2 |
Whole mAb ADC |
||
Pre-Made Azintuxizumab biosimilar, Whole mAb ADC, Anti-SLAMF7/CS1 Antibody: Anti-19A/CD319/CRACC therapeutic antibody |
Azintuxizumab |
NA |
SLAMF7/CS1 |
Whole mAb ADC |
||
Pre-Made Belantamab biosimilar, Whole mAb ADC, Anti-TNFRSF17 Antibody: Anti-BCM/BCMA/CD269/TNFRSF13A therapeutic antibody |
Belantamab |
NA |
TNFRSF17 |
Whole mAb ADC |
||
Pre-Made Brentuximab biosimilar, Whole mAb ADC, Anti-TNFRSF8 Antibody: Anti-CD30/Ki-1/D1S166E therapeutic antibody |
Brentuximab |
NA |
TNFRSF8 |
Whole mAb ADC |
||
Pre-Made Camidanlumab biosimilar, Whole mAb ADC, Anti-IL2RA Antibody: Anti-CD25/IDDM10/IL2R/IMD41/TCGFR/p55 therapeutic antibody |
Camidanlumab |
NA |
IL2RA |
Whole mAb ADC |
||
Pre-Made Cantuzumab biosimilar, Whole mAb ADC, Anti-MUC1 Antibody: Anti-ADMCKD/ADMCKD1/ADTKD2/CA 15-3/CD227/Ca15-3/EMA/H23AG/KL-6/MAM6/MCD/MCKD/MCKD1/SEC/X/ZD/PEM/PEMT/PUM therapeutic antibody |
Cantuzumab |
NA |
MUC1 |
Whole mAb ADC |
||
Pre-Made Cofetuzumab biosimilar, Whole mAb ADC, Anti-PTK7 Antibody: Anti-CCK-4/CCK4 therapeutic antibody |
Cofetuzumab |
NA |
PTK7 |
Whole mAb ADC |
||
Pre-Made Coltuximab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Coltuximab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Denintuzumab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Denintuzumab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Depatuxizumab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Depatuxizumab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Disitamab biosimilar, Whole mAb ADC, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2 therapeutic antibody |
Disitamab |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Enapotamab biosimilar, Whole mAb ADC, Anti-AXL Antibody: Anti-ARK/UFO/JTK11/Tyro7 therapeutic antibody |
Enapotamab |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Enfortumab biosimilar, Whole mAb ADC, Anti-PVRL4/NECTIN4 Antibody: Anti-EDSS1/LNIR/PRR4/nectin-4 therapeutic antibody |
Enfortumab |
NA |
PVRL4 |
Whole mAb ADC |
||
Pre-Made Gemtuzumab biosimilar, Whole mAb ADC, Anti-CD33 Antibody: Anti-p67/SIGLEC3/SIGLEC-3 therapeutic antibody |
Gemtuzumab |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Glembatumumab biosimilar, Whole mAb ADC, Anti-GPNMB Antibody: Anti-HGFIN/NMB/PLCA3 therapeutic antibody |
Glembatumumab |
NA |
GPNMB |
Whole mAb ADC |
||
Pre-Made Iladatuzumab biosimilar, Whole mAb ADC, Anti-CD79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody |
Iladatuzumab |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Indatuximab biosimilar, Whole mAb ADC, Anti-SDC1 Antibody: Anti-SDC/CD138/SYND1/syndecan therapeutic antibody |
Indatuximab |
NA |
SDC1 |
Whole mAb ADC |
||
Pre-Made Indusatumab biosimilar, Whole mAb ADC, Anti-GUCY2C Antibody: Anti-DIAR6/GC-C/GUC2C/MECILIL/STAR therapeutic antibody |
Indusatumab |
NA |
GUCY2C |
Whole mAb ADC |
||
Pre-Made Inotuzumab biosimilar, Whole mAb ADC, Anti-CD22 Antibody: Anti-SIGLEC2/SIGLEC-2 therapeutic antibody |
Inotuzumab |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Labetuzumab biosimilar, Whole mAb ADC, Anti-CEACAM5/CD66e Antibody: Anti-CEA therapeutic antibody |
Labetuzumab |
NA |
CEACAM5 |
Whole mAb ADC |
||
Pre-Made Ladiratuzumab biosimilar, Whole mAb ADC, Anti-SLC39A6 Antibody: Anti-LIV-1/LIV1/ZIP6 therapeutic antibody |
Ladiratuzumab |
NA |
SLC39A6 |
Whole mAb ADC |
||
Pre-Made Laprituximab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Laprituximab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lifastuzumab biosimilar, Whole mAb ADC, Anti-SLC34A2 Antibody: Anti-NAPI-3B/NAPI-IIb/NPTIIb/PULAM therapeutic antibody |
Lifastuzumab |
NA |
SLC34A2 |
Whole mAb ADC |
||
Pre-Made Lilotomab biosimilar, Whole mAb ADC, Anti-CD37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody |
Lilotomab |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Loncastuximab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Loncastuximab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Lorvotuzumab biosimilar, Whole mAb ADC, Anti-NCAM1 Antibody: Anti-CD56/NCAM/MSK39 therapeutic antibody |
Lorvotuzumab |
NA |
NCAM1 |
Whole mAb ADC |
||
Pre-Made Losatuxizumab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Losatuxizumab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lupartumab biosimilar, Whole mAb ADC, Anti-LYPD3 Antibody: Anti-C4.4A therapeutic antibody |
Lupartumab |
NA |
LYPD3 |
Whole mAb ADC |
||
Pre-Made Milatuzumab biosimilar, Whole mAb, Anti-CD74 Antibody: Anti-CLIP/DHLAG/HLADG/II/Ia-GAMMA/p33 therapeutic antibody |
Milatuzumab |
NA |
CD74 |
Whole mAb ADC |
||
Pre-Made Mirvetuximab biosimilar, Whole mAb ADC, Anti-FOLR1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody |
Mirvetuximab |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Naratuximab biosimilar, Whole mAb ADC, Anti-CD37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody |
Naratuximab |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Pelgifatamab biosimilar, Whole mAb ADC, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody |
Pelgifatamab |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Pinatuzumab biosimilar, Whole mAb ADC, Anti-CD22 Antibody: Anti-SIGLEC2/SIGLEC-2 therapeutic antibody |
Pinatuzumab |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Polatuzumab biosimilar, Whole mAb ADC, Anti-CD79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody |
Polatuzumab |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Rolinsatamab biosimilar, Whole mAb ADC, Anti-PRLR Antibody: Anti-HPRL/MFAB/RI-PRLR/hPRLrI therapeutic antibody |
Rolinsatamab |
NA |
PRLR |
Whole mAb ADC |
||
Pre-Made Rovalpituzumab biosimilar, Whole mAb ADC, Anti-DLL3 Antibody: Anti-SCDO1 therapeutic antibody |
Rovalpituzumab |
NA |
DLL3 |
Whole mAb ADC |
||
Pre-Made Sacituzumab biosimilar, Whole mAb ADC, Anti-TACSTD2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody |
Sacituzumab |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Samrotamab biosimilar, Whole mAb ADC, Anti-LRRC15 Antibody: Anti-LIB therapeutic antibody |
Samrotamab |
NA |
LRRC15 |
Whole mAb ADC |
||
Pre-Made Serclutamab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Serclutamab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Sirtratumab biosimilar, Whole mAb ADC, Anti-SLITRK6 Antibody: Anti-DFNMYP therapeutic antibody |
Sirtratumab |
NA |
SLITRK6 |
Whole mAb ADC |
||
Pre-Made Sofituzumab biosimilar, Whole mAb ADC, Anti-MUC16 Antibody: Anti-CA125 therapeutic antibody |
Sofituzumab |
NA |
MUC16 |
Whole mAb ADC |
||
Pre-Made Tabituximab biosimilar, Whole mAb ADC, Anti-FZD10 Antibody: Anti-CD350/FZ-10/Fz10/FzE7/hFz10 therapeutic antibody |
Tabituximab |
NA |
FZD10 |
Whole mAb ADC |
||
Pre-Made Tamrintamab biosimilar, Whole mAb ADC, Anti-DPEP3 Antibody: Anti-MBD3 therapeutic antibody |
Tamrintamab |
NA |
DPEP3 |
Whole mAb ADC |
||
Pre-Made Telisotuzumab biosimilar, Whole mAb ADC, Anti-MET Antibody: Anti-AUTS9/DFNB97/HGFR/RCCP2/c-Met therapeutic antibody |
Telisotuzumab |
NA |
MET |
Whole mAb ADC |
||
Pre-Made Vadastuximab biosimilar, Whole mAb ADC, Anti-CD33 Antibody: Anti-p67/SIGLEC3/SIGLEC-3 therapeutic antibody |
Vadastuximab |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Vandortuzumab biosimilar, Whole mAb ADC, Anti-STEAP1 Antibody: Anti-PRSS24/STEAP therapeutic antibody |
Vandortuzumab |
NA |
STEAP1 |
Whole mAb ADC |
||
Pre-Made Vorsetuzumab biosimilar, Whole mAb ADC, Anti-CD70/CD27-L Antibody: Anti-CD27L/LPFS3/CD27LG/TNFSF7/TNLG8A therapeutic antibody |
Vorsetuzumab |
NA |
CD70 |
Whole mAb ADC |
||
Pre-Made Tabituximab Barzuxetan Biosimilar, Whole Mab Adc, Anti-Fzd10 Antibody: Anti-CD350/FZ-10/Fz10/FzE7/hFz10 therapeutic antibody Drug Conjugate |
tabituximab barzuxetan |
NA |
FZD10 |
Whole mAb ADC |
||
Pre-Made Tamrintamab Pamozirine Biosimilar, Whole Mab Adc, Anti-Dpep3 Antibody: Anti-MBD3 therapeutic antibody Drug Conjugate |
tamrintamab pamozirine |
NA |
DPEP3 |
Whole mAb ADC |
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




