Site specific biological coupling of engineered antibodies and Enzymatic method
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

Site specific biological coupling of engineered antibodies
Advances in bioorthogonal chemistry and protein engineering contribute to the generation of more uniform ADCs. Although there are many attachment methods available on natural monoclonal antibodies, site-specific biological coupling on engineered antibodies can more effectively control Dar and avoid changing the affinity for antigen binding. In this way, natural or unnatural amino acids are added at some positions to obtain homogeneous products with excellent pharmacokinetic and pharmacodynamic characteristics.
Enzymatic method
The attachment of payloads can be achieved in a very selective manner by inserting specific amino acid tags into the antibody sequence. These tags are recognized by specific enzymes, such as formylglycine generating enzyme (FGE), microbial transglutaminase (MTG), transpeptidase or tyrosinase, so that site-specific coupling can be performed.
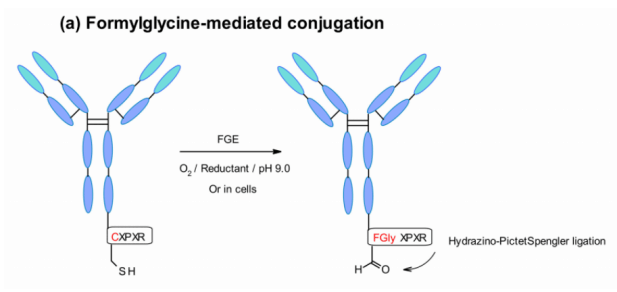
Aaron et al. Explored a new site-specific coupling of aldehyde labeled proteins. The technique utilizes a gene encoded pentapeptide sequence (Cys-X-Pro-X-Arg), in which cysteine residues are recognized by FGE and co translated and oxidized to formylglycine during protein expression in cells. In this way, the engineered antibody is selectively coupled with aldehyde specific linker by hips (hydrazino Pictet – Spengler) chemical method.
Microbial transglutaminase (MTGase) strategies are also often developed for location-specific coupling. MTGase catalyzes the formation of peptide bonds between the glutamine side chain at the 295 position of the glycosylated antibody and the primary amine of the substrate. Compared with other enzyme strategies, MTG is a flexible technology and does not require peptide donors to achieve coupling. As long as the acyl receptor contains a primary amine, there is no structural restriction.
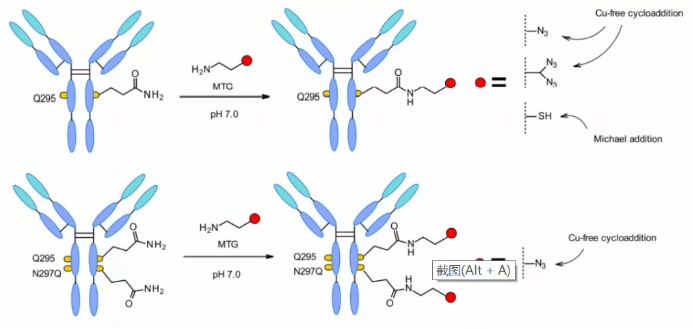
Glutamine residues naturally exist in the Fc region of each heavy chain of the McAb. After deglycosylation at position 295, glutamine residues are coupled through MTGase mediated reaction to produce a uniform ADC with Dar = 2. In order to improve the efficiency, the linker with branched chain can be coupled to double the Dar, and the mutation of asparagine at position 297 to glutamine can also increase the DAR.
NBE therapeutics developed S-based Staphylococcus aureus transpeptidase A-mediated coupling. Their strategy uses transpeptidase a (srtA) to cut the amide bond between threonine and glycine residues in the motif of lpxtg (X = any amino acid) pentapeptide. It then catalyzes the coupling of glycine related payloads with the newly formed C-terminal to form peptide bonds at physiological temperature and pH.
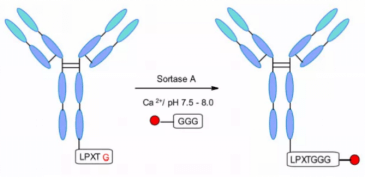
The method was applied to different antibodies, such as anti-CD30 and anti-HER2, and maytanine and MMAE were coupled with 5-glycine labeled linkers. Both ADCs showed similar in vitro cytotoxicity to classical coupling. Trastuzumab maytanine produced by enzymatic method completely matched kadcyla in vivo test.In another example, the ADC of efficient anthracycline toxin derivative pnu-159682 was generated by transpeptidase method. Interestingly, through this technology, the coupling efficiency is even higher than that of adcetris and kadcyla analogues. In addition, the prepared pnu-159682 ADC has high stability in vitro and in vivo, and shows more efficacy than the ADC containing tubulin targeting payload.
Product List
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Anetumab biosimilar, Whole mAb ADC, Anti-MSLN Antibody: Anti-MPF/SMRP therapeutic antibody |
Anetumab |
NA |
MSLN |
Whole mAb ADC |
||
Pre-Made Aprutumab biosimilar, Whole mAb ADC, Anti-FGFR2 Antibody: Anti-BBDS/BEK/BFR-1/CD332/CEK3/CFD1/ECT1/JWS/K-SAM/KGFR/TK14/TK25 therapeutic antibody |
Aprutumab |
NA |
FGFR2 |
Whole mAb ADC |
||
Pre-Made Azintuxizumab biosimilar, Whole mAb ADC, Anti-SLAMF7/CS1 Antibody: Anti-19A/CD319/CRACC therapeutic antibody |
Azintuxizumab |
NA |
SLAMF7/CS1 |
Whole mAb ADC |
||
Pre-Made Belantamab biosimilar, Whole mAb ADC, Anti-TNFRSF17 Antibody: Anti-BCM/BCMA/CD269/TNFRSF13A therapeutic antibody |
Belantamab |
NA |
TNFRSF17 |
Whole mAb ADC |
||
Pre-Made Brentuximab biosimilar, Whole mAb ADC, Anti-TNFRSF8 Antibody: Anti-CD30/Ki-1/D1S166E therapeutic antibody |
Brentuximab |
NA |
TNFRSF8 |
Whole mAb ADC |
||
Pre-Made Camidanlumab biosimilar, Whole mAb ADC, Anti-IL2RA Antibody: Anti-CD25/IDDM10/IL2R/IMD41/TCGFR/p55 therapeutic antibody |
Camidanlumab |
NA |
IL2RA |
Whole mAb ADC |
||
Pre-Made Cantuzumab biosimilar, Whole mAb ADC, Anti-MUC1 Antibody: Anti-ADMCKD/ADMCKD1/ADTKD2/CA 15-3/CD227/Ca15-3/EMA/H23AG/KL-6/MAM6/MCD/MCKD/MCKD1/SEC/X/ZD/PEM/PEMT/PUM therapeutic antibody |
Cantuzumab |
NA |
MUC1 |
Whole mAb ADC |
||
Pre-Made Cofetuzumab biosimilar, Whole mAb ADC, Anti-PTK7 Antibody: Anti-CCK-4/CCK4 therapeutic antibody |
Cofetuzumab |
NA |
PTK7 |
Whole mAb ADC |
||
Pre-Made Coltuximab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Coltuximab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Denintuzumab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Denintuzumab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Depatuxizumab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Depatuxizumab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Disitamab biosimilar, Whole mAb ADC, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2 therapeutic antibody |
Disitamab |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Enapotamab biosimilar, Whole mAb ADC, Anti-AXL Antibody: Anti-ARK/UFO/JTK11/Tyro7 therapeutic antibody |
Enapotamab |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Enfortumab biosimilar, Whole mAb ADC, Anti-PVRL4/NECTIN4 Antibody: Anti-EDSS1/LNIR/PRR4/nectin-4 therapeutic antibody |
Enfortumab |
NA |
PVRL4 |
Whole mAb ADC |
||
Pre-Made Gemtuzumab biosimilar, Whole mAb ADC, Anti-CD33 Antibody: Anti-p67/SIGLEC3/SIGLEC-3 therapeutic antibody |
Gemtuzumab |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Glembatumumab biosimilar, Whole mAb ADC, Anti-GPNMB Antibody: Anti-HGFIN/NMB/PLCA3 therapeutic antibody |
Glembatumumab |
NA |
GPNMB |
Whole mAb ADC |
||
Pre-Made Iladatuzumab biosimilar, Whole mAb ADC, Anti-CD79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody |
Iladatuzumab |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Indatuximab biosimilar, Whole mAb ADC, Anti-SDC1 Antibody: Anti-SDC/CD138/SYND1/syndecan therapeutic antibody |
Indatuximab |
NA |
SDC1 |
Whole mAb ADC |
||
Pre-Made Indusatumab biosimilar, Whole mAb ADC, Anti-GUCY2C Antibody: Anti-DIAR6/GC-C/GUC2C/MECILIL/STAR therapeutic antibody |
Indusatumab |
NA |
GUCY2C |
Whole mAb ADC |
||
Pre-Made Inotuzumab biosimilar, Whole mAb ADC, Anti-CD22 Antibody: Anti-SIGLEC2/SIGLEC-2 therapeutic antibody |
Inotuzumab |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Labetuzumab biosimilar, Whole mAb ADC, Anti-CEACAM5/CD66e Antibody: Anti-CEA therapeutic antibody |
Labetuzumab |
NA |
CEACAM5 |
Whole mAb ADC |
||
Pre-Made Ladiratuzumab biosimilar, Whole mAb ADC, Anti-SLC39A6 Antibody: Anti-LIV-1/LIV1/ZIP6 therapeutic antibody |
Ladiratuzumab |
NA |
SLC39A6 |
Whole mAb ADC |
||
Pre-Made Laprituximab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Laprituximab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lifastuzumab biosimilar, Whole mAb ADC, Anti-SLC34A2 Antibody: Anti-NAPI-3B/NAPI-IIb/NPTIIb/PULAM therapeutic antibody |
Lifastuzumab |
NA |
SLC34A2 |
Whole mAb ADC |
||
Pre-Made Lilotomab biosimilar, Whole mAb ADC, Anti-CD37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody |
Lilotomab |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Loncastuximab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Loncastuximab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Lorvotuzumab biosimilar, Whole mAb ADC, Anti-NCAM1 Antibody: Anti-CD56/NCAM/MSK39 therapeutic antibody |
Lorvotuzumab |
NA |
NCAM1 |
Whole mAb ADC |
||
Pre-Made Losatuxizumab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Losatuxizumab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lupartumab biosimilar, Whole mAb ADC, Anti-LYPD3 Antibody: Anti-C4.4A therapeutic antibody |
Lupartumab |
NA |
LYPD3 |
Whole mAb ADC |
||
Pre-Made Milatuzumab biosimilar, Whole mAb, Anti-CD74 Antibody: Anti-CLIP/DHLAG/HLADG/II/Ia-GAMMA/p33 therapeutic antibody |
Milatuzumab |
NA |
CD74 |
Whole mAb ADC |
||
Pre-Made Mirvetuximab biosimilar, Whole mAb ADC, Anti-FOLR1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody |
Mirvetuximab |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Naratuximab biosimilar, Whole mAb ADC, Anti-CD37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody |
Naratuximab |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Pelgifatamab biosimilar, Whole mAb ADC, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody |
Pelgifatamab |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Pinatuzumab biosimilar, Whole mAb ADC, Anti-CD22 Antibody: Anti-SIGLEC2/SIGLEC-2 therapeutic antibody |
Pinatuzumab |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Polatuzumab biosimilar, Whole mAb ADC, Anti-CD79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody |
Polatuzumab |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Rolinsatamab biosimilar, Whole mAb ADC, Anti-PRLR Antibody: Anti-HPRL/MFAB/RI-PRLR/hPRLrI therapeutic antibody |
Rolinsatamab |
NA |
PRLR |
Whole mAb ADC |
||
Pre-Made Rovalpituzumab biosimilar, Whole mAb ADC, Anti-DLL3 Antibody: Anti-SCDO1 therapeutic antibody |
Rovalpituzumab |
NA |
DLL3 |
Whole mAb ADC |
||
Pre-Made Sacituzumab biosimilar, Whole mAb ADC, Anti-TACSTD2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody |
Sacituzumab |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Samrotamab biosimilar, Whole mAb ADC, Anti-LRRC15 Antibody: Anti-LIB therapeutic antibody |
Samrotamab |
NA |
LRRC15 |
Whole mAb ADC |
||
Pre-Made Serclutamab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Serclutamab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Sirtratumab biosimilar, Whole mAb ADC, Anti-SLITRK6 Antibody: Anti-DFNMYP therapeutic antibody |
Sirtratumab |
NA |
SLITRK6 |
Whole mAb ADC |
||
Pre-Made Sofituzumab biosimilar, Whole mAb ADC, Anti-MUC16 Antibody: Anti-CA125 therapeutic antibody |
Sofituzumab |
NA |
MUC16 |
Whole mAb ADC |
||
Pre-Made Tabituximab biosimilar, Whole mAb ADC, Anti-FZD10 Antibody: Anti-CD350/FZ-10/Fz10/FzE7/hFz10 therapeutic antibody |
Tabituximab |
NA |
FZD10 |
Whole mAb ADC |
||
Pre-Made Tamrintamab biosimilar, Whole mAb ADC, Anti-DPEP3 Antibody: Anti-MBD3 therapeutic antibody |
Tamrintamab |
NA |
DPEP3 |
Whole mAb ADC |
||
Pre-Made Telisotuzumab biosimilar, Whole mAb ADC, Anti-MET Antibody: Anti-AUTS9/DFNB97/HGFR/RCCP2/c-Met therapeutic antibody |
Telisotuzumab |
NA |
MET |
Whole mAb ADC |
||
Pre-Made Vadastuximab biosimilar, Whole mAb ADC, Anti-CD33 Antibody: Anti-p67/SIGLEC3/SIGLEC-3 therapeutic antibody |
Vadastuximab |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Vandortuzumab biosimilar, Whole mAb ADC, Anti-STEAP1 Antibody: Anti-PRSS24/STEAP therapeutic antibody |
Vandortuzumab |
NA |
STEAP1 |
Whole mAb ADC |
||
Pre-Made Vorsetuzumab biosimilar, Whole mAb ADC, Anti-CD70/CD27-L Antibody: Anti-CD27L/LPFS3/CD27LG/TNFSF7/TNLG8A therapeutic antibody |
Vorsetuzumab |
NA |
CD70 |
Whole mAb ADC |
||
Pre-Made Tabituximab Barzuxetan Biosimilar, Whole Mab Adc, Anti-Fzd10 Antibody: Anti-CD350/FZ-10/Fz10/FzE7/hFz10 therapeutic antibody Drug Conjugate |
tabituximab barzuxetan |
NA |
FZD10 |
Whole mAb ADC |
||
Pre-Made Tamrintamab Pamozirine Biosimilar, Whole Mab Adc, Anti-Dpep3 Antibody: Anti-MBD3 therapeutic antibody Drug Conjugate |
tamrintamab pamozirine |
NA |
DPEP3 |
Whole mAb ADC |
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




