Product data of ADC
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

Product List
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Anetumab biosimilar, Whole mAb ADC, Anti-MSLN Antibody: Anti-MPF/SMRP therapeutic antibody |
Anetumab |
NA |
MSLN |
Whole mAb ADC |
||
Pre-Made Aprutumab biosimilar, Whole mAb ADC, Anti-FGFR2 Antibody: Anti-BBDS/BEK/BFR-1/CD332/CEK3/CFD1/ECT1/JWS/K-SAM/KGFR/TK14/TK25 therapeutic antibody |
Aprutumab |
NA |
FGFR2 |
Whole mAb ADC |
||
Pre-Made Azintuxizumab biosimilar, Whole mAb ADC, Anti-SLAMF7/CS1 Antibody: Anti-19A/CD319/CRACC therapeutic antibody |
Azintuxizumab |
NA |
SLAMF7/CS1 |
Whole mAb ADC |
||
Pre-Made Belantamab biosimilar, Whole mAb ADC, Anti-TNFRSF17 Antibody: Anti-BCM/BCMA/CD269/TNFRSF13A therapeutic antibody |
Belantamab |
NA |
TNFRSF17 |
Whole mAb ADC |
||
Pre-Made Brentuximab biosimilar, Whole mAb ADC, Anti-TNFRSF8 Antibody: Anti-CD30/Ki-1/D1S166E therapeutic antibody |
Brentuximab |
NA |
TNFRSF8 |
Whole mAb ADC |
||
Pre-Made Camidanlumab biosimilar, Whole mAb ADC, Anti-IL2RA Antibody: Anti-CD25/IDDM10/IL2R/IMD41/TCGFR/p55 therapeutic antibody |
Camidanlumab |
NA |
IL2RA |
Whole mAb ADC |
||
Pre-Made Cantuzumab biosimilar, Whole mAb ADC, Anti-MUC1 Antibody: Anti-ADMCKD/ADMCKD1/ADTKD2/CA 15-3/CD227/Ca15-3/EMA/H23AG/KL-6/MAM6/MCD/MCKD/MCKD1/SEC/X/ZD/PEM/PEMT/PUM therapeutic antibody |
Cantuzumab |
NA |
MUC1 |
Whole mAb ADC |
||
Pre-Made Cofetuzumab biosimilar, Whole mAb ADC, Anti-PTK7 Antibody: Anti-CCK-4/CCK4 therapeutic antibody |
Cofetuzumab |
NA |
PTK7 |
Whole mAb ADC |
||
Pre-Made Coltuximab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Coltuximab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Denintuzumab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Denintuzumab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Depatuxizumab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Depatuxizumab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Disitamab biosimilar, Whole mAb ADC, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2 therapeutic antibody |
Disitamab |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Enapotamab biosimilar, Whole mAb ADC, Anti-AXL Antibody: Anti-ARK/UFO/JTK11/Tyro7 therapeutic antibody |
Enapotamab |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Enfortumab biosimilar, Whole mAb ADC, Anti-PVRL4/NECTIN4 Antibody: Anti-EDSS1/LNIR/PRR4/nectin-4 therapeutic antibody |
Enfortumab |
NA |
PVRL4 |
Whole mAb ADC |
||
Pre-Made Gemtuzumab biosimilar, Whole mAb ADC, Anti-CD33 Antibody: Anti-p67/SIGLEC3/SIGLEC-3 therapeutic antibody |
Gemtuzumab |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Glembatumumab biosimilar, Whole mAb ADC, Anti-GPNMB Antibody: Anti-HGFIN/NMB/PLCA3 therapeutic antibody |
Glembatumumab |
NA |
GPNMB |
Whole mAb ADC |
||
Pre-Made Iladatuzumab biosimilar, Whole mAb ADC, Anti-CD79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody |
Iladatuzumab |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Indatuximab biosimilar, Whole mAb ADC, Anti-SDC1 Antibody: Anti-SDC/CD138/SYND1/syndecan therapeutic antibody |
Indatuximab |
NA |
SDC1 |
Whole mAb ADC |
||
Pre-Made Indusatumab biosimilar, Whole mAb ADC, Anti-GUCY2C Antibody: Anti-DIAR6/GC-C/GUC2C/MECILIL/STAR therapeutic antibody |
Indusatumab |
NA |
GUCY2C |
Whole mAb ADC |
||
Pre-Made Inotuzumab biosimilar, Whole mAb ADC, Anti-CD22 Antibody: Anti-SIGLEC2/SIGLEC-2 therapeutic antibody |
Inotuzumab |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Labetuzumab biosimilar, Whole mAb ADC, Anti-CEACAM5/CD66e Antibody: Anti-CEA therapeutic antibody |
Labetuzumab |
NA |
CEACAM5 |
Whole mAb ADC |
||
Pre-Made Ladiratuzumab biosimilar, Whole mAb ADC, Anti-SLC39A6 Antibody: Anti-LIV-1/LIV1/ZIP6 therapeutic antibody |
Ladiratuzumab |
NA |
SLC39A6 |
Whole mAb ADC |
||
Pre-Made Laprituximab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Laprituximab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lifastuzumab biosimilar, Whole mAb ADC, Anti-SLC34A2 Antibody: Anti-NAPI-3B/NAPI-IIb/NPTIIb/PULAM therapeutic antibody |
Lifastuzumab |
NA |
SLC34A2 |
Whole mAb ADC |
||
Pre-Made Lilotomab biosimilar, Whole mAb ADC, Anti-CD37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody |
Lilotomab |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Loncastuximab biosimilar, Whole mAb ADC, Anti-CD19 Antibody: Anti-B4/CVID3 therapeutic antibody |
Loncastuximab |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Lorvotuzumab biosimilar, Whole mAb ADC, Anti-NCAM1 Antibody: Anti-CD56/NCAM/MSK39 therapeutic antibody |
Lorvotuzumab |
NA |
NCAM1 |
Whole mAb ADC |
||
Pre-Made Losatuxizumab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Losatuxizumab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lupartumab biosimilar, Whole mAb ADC, Anti-LYPD3 Antibody: Anti-C4.4A therapeutic antibody |
Lupartumab |
NA |
LYPD3 |
Whole mAb ADC |
||
Pre-Made Milatuzumab biosimilar, Whole mAb, Anti-CD74 Antibody: Anti-CLIP/DHLAG/HLADG/II/Ia-GAMMA/p33 therapeutic antibody |
Milatuzumab |
NA |
CD74 |
Whole mAb ADC |
||
Pre-Made Mirvetuximab biosimilar, Whole mAb ADC, Anti-FOLR1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody |
Mirvetuximab |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Naratuximab biosimilar, Whole mAb ADC, Anti-CD37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody |
Naratuximab |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Pelgifatamab biosimilar, Whole mAb ADC, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody |
Pelgifatamab |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Pinatuzumab biosimilar, Whole mAb ADC, Anti-CD22 Antibody: Anti-SIGLEC2/SIGLEC-2 therapeutic antibody |
Pinatuzumab |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Polatuzumab biosimilar, Whole mAb ADC, Anti-CD79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody |
Polatuzumab |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Rolinsatamab biosimilar, Whole mAb ADC, Anti-PRLR Antibody: Anti-HPRL/MFAB/RI-PRLR/hPRLrI therapeutic antibody |
Rolinsatamab |
NA |
PRLR |
Whole mAb ADC |
||
Pre-Made Rovalpituzumab biosimilar, Whole mAb ADC, Anti-DLL3 Antibody: Anti-SCDO1 therapeutic antibody |
Rovalpituzumab |
NA |
DLL3 |
Whole mAb ADC |
||
Pre-Made Sacituzumab biosimilar, Whole mAb ADC, Anti-TACSTD2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody |
Sacituzumab |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Samrotamab biosimilar, Whole mAb ADC, Anti-LRRC15 Antibody: Anti-LIB therapeutic antibody |
Samrotamab |
NA |
LRRC15 |
Whole mAb ADC |
||
Pre-Made Serclutamab biosimilar, Whole mAb ADC, Anti-EGFR Antibody: Anti-ERBB/ERRP/HER1/mENA/ERBB1/PIG61/NISBD2 therapeutic antibody |
Serclutamab |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Sirtratumab biosimilar, Whole mAb ADC, Anti-SLITRK6 Antibody: Anti-DFNMYP therapeutic antibody |
Sirtratumab |
NA |
SLITRK6 |
Whole mAb ADC |
||
Pre-Made Sofituzumab biosimilar, Whole mAb ADC, Anti-MUC16 Antibody: Anti-CA125 therapeutic antibody |
Sofituzumab |
NA |
MUC16 |
Whole mAb ADC |
||
Pre-Made Tabituximab biosimilar, Whole mAb ADC, Anti-FZD10 Antibody: Anti-CD350/FZ-10/Fz10/FzE7/hFz10 therapeutic antibody |
Tabituximab |
NA |
FZD10 |
Whole mAb ADC |
||
Pre-Made Tamrintamab biosimilar, Whole mAb ADC, Anti-DPEP3 Antibody: Anti-MBD3 therapeutic antibody |
Tamrintamab |
NA |
DPEP3 |
Whole mAb ADC |
||
Pre-Made Telisotuzumab biosimilar, Whole mAb ADC, Anti-MET Antibody: Anti-AUTS9/DFNB97/HGFR/RCCP2/c-Met therapeutic antibody |
Telisotuzumab |
NA |
MET |
Whole mAb ADC |
||
Pre-Made Vadastuximab biosimilar, Whole mAb ADC, Anti-CD33 Antibody: Anti-p67/SIGLEC3/SIGLEC-3 therapeutic antibody |
Vadastuximab |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Vandortuzumab biosimilar, Whole mAb ADC, Anti-STEAP1 Antibody: Anti-PRSS24/STEAP therapeutic antibody |
Vandortuzumab |
NA |
STEAP1 |
Whole mAb ADC |
||
Pre-Made Vorsetuzumab biosimilar, Whole mAb ADC, Anti-CD70/CD27-L Antibody: Anti-CD27L/LPFS3/CD27LG/TNFSF7/TNLG8A therapeutic antibody |
Vorsetuzumab |
NA |
CD70 |
Whole mAb ADC |
||
Pre-Made Tabituximab Barzuxetan Biosimilar, Whole Mab Adc, Anti-Fzd10 Antibody: Anti-CD350/FZ-10/Fz10/FzE7/hFz10 therapeutic antibody Drug Conjugate |
tabituximab barzuxetan |
NA |
FZD10 |
Whole mAb ADC |
||
Pre-Made Tamrintamab Pamozirine Biosimilar, Whole Mab Adc, Anti-Dpep3 Antibody: Anti-MBD3 therapeutic antibody Drug Conjugate |
tamrintamab pamozirine |
NA |
DPEP3 |
Whole mAb ADC |
Case study: Product data of Antibody-drug conjugate (ADC)
| Antibody | Payload | QC |
|---|---|---|
| Ab-001 | VCMMAE | SDS-PAGE(reducing and non-reducing) |
| Human IgG1 Control | DAR | |
| Ab-002 | Cytotoxity assay |
First, we used a higher dose of reducing agent (TCEP) and small toxic molecule (vcmmae) to ensure the success of coupling. SDS-PAGE (reducing DTT & non reducing DTT) results showed that we successfully linked MMAE to the antibody. Here you are the conjugate manufacturing.
2.1 human IgG1-ADC: SDS-PAGE (reducing) and SDS-PAGE (non-reducing)
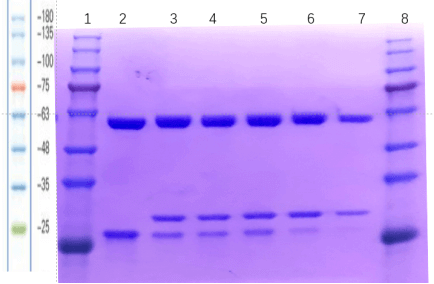
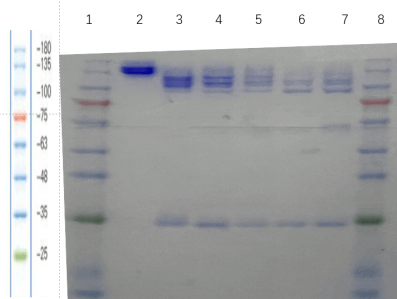
2.2 DAR detect
In this batch, we selected 5.4 equivalent TCEP and 10.8 equivalent VCMMAE groups for DAR detection. The DAR of our sample is almost the same as that of the standard sample, but the integrity of the antibody result is not as good as that of the standard sample.
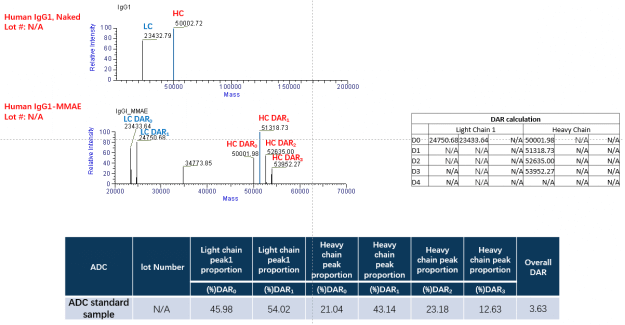
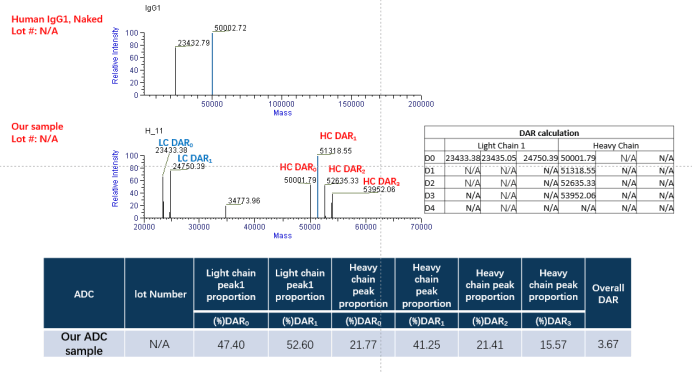
Figure. The DAR detect of our ADC sample by LC-MS. At the beginning of this project, to ensure the antibody can conjugate with payload(MMAE), the dose of TCEP and MMAE is high. However, we find the dose of TCEP cannot be high, otherwise the damage to the antibody structure is more severe.
2.3 cytotoxity assay
At next step, we did cytotoxity assay to observe the toxic effect of ADC on 293 cells. At the same time, considering the storage and transportation of ADC, we lyophilized ADC and compared the effect of non-lyophilized on ADC cytotoxicity.
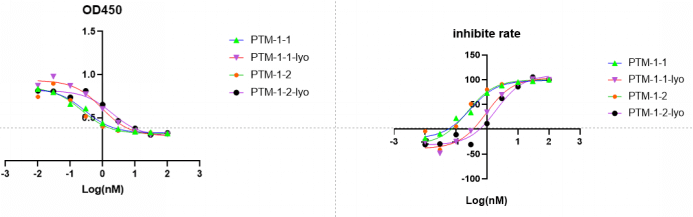
Obviously, compared with the blank group, ADC had a significant inhibitory effect on the growth of 293 cells. Moreover, lyophilized can reduce the inhibition of ADC on cell growth.
The results show that when the equivalent of reducing agent TCEP is 0.4 and 1.5, the results do not have regularity, which may be caused by the limited opening of disulfide bond and the limited coupling of small molecules. However, when the equivalent of TCEP is 3, the results can match the previous PTM-1-ADC (5.4t5.4v & 5.4t10.8). We also deal 293-ko cell line with PTM-1-ADC, obviously, there is no regular relationship between cell death and ADC dose.
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




