DOI: 10.1021/acs.analchem.0c01975[2] https://med.unr.edu/ddl/technology/lateral-flow-immunoassay[3] Cate, David; Hsieh, Helen; Glukhova, Veronika; Bishop, Joshua D; Hermansky, H Gleda; Barrios-Lopez, Brianda; et al. (2020): Antibody Screening Results for Anti-Nucleocapsid Antibodies Towards the Development of a SARS-CoV-2 Nucleocapsid Protein Antigen Detecting Lateral Flow Assay. ChemRxiv. Preprint. https://doi.org/10.26434/chemrxiv.12709538.v1
GeneMedi’s Antigens and Antibodies for COVID-19 Rapid Test and Immunoassay
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

The ongoing worldwide severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic has a tremendous influence on public health. There is an urgent need for developing accurate detection technologies for COVID-19 pandemic. Several diagnostic strategies are available to identify or rule out current infection, identify people in need of care escalation, or to test for past infection and immune response. Point-of-care Lateral flow assays (LFAs) and molecular tests to detect current SARS-CoV-2 infection have the potential to allow earlier detection and isolation of confirmed cases compared to laboratory-based diagnostic methods, with the aim of reducing household and community transmission.
As an innovator in biologics, GeneMedi takes response rapidly to the COVID-19 pandemic in COVID-19 diagnostics strategy, solution and product discovery and development. GeneMedi develops COVID-19 related antigens and antibodies as core ingredients of COVID-19 test kit.
Antigens including Nucleocapsid (N protein), Spike protein (S protein, S1+S2 ECD), Spike protein (S1 protein), spike RBD protein, Envelope (E protein), 3C-like Proteinase (Mpro), RdRP, et,al. These recombinant antigens are available and ready-to-use on GeneMedi:
https://www.GeneMedi.com/i/recombinant-2019-ncov-antigens-reagents
Antibodies including validated Spike antibodies and NP antibodies in pair against COVID-19.
https://www.GeneMedi.com/i/2019ncov-antibody
GeneMedi’s COVID-19 diagnostics solution and products have been validated and applied by global diagnostic companies, and highly evaluated by them. In addition, many articles have cited GeneMedi’s COVID-19 products and given high comments.
Antigens for COVID-19 Rapid Test and Antibodies Screening
Grant et al[1]. described a half-strip Lateral flow assays (LFAs), which is a test format frequently utilized as the first step in assay development for a “full” LFA. Reverse transcription polymerase chain reaction (RT-PCR) based on oral swabs was used for confirmation of SARS-CoV-2 infection. However, high false negative rates, implementation costs and logistical problems with reagents during the global SARS-CoV-2 pandemic have hindered its universal on demand adoption. Lateral flow assays (LFAs) represent a class of diagnostic that, if sufficiently clinically sensitive, may fill many of the gaps in the current RT-PCR testing regime, especially in low- and middle-income countries.
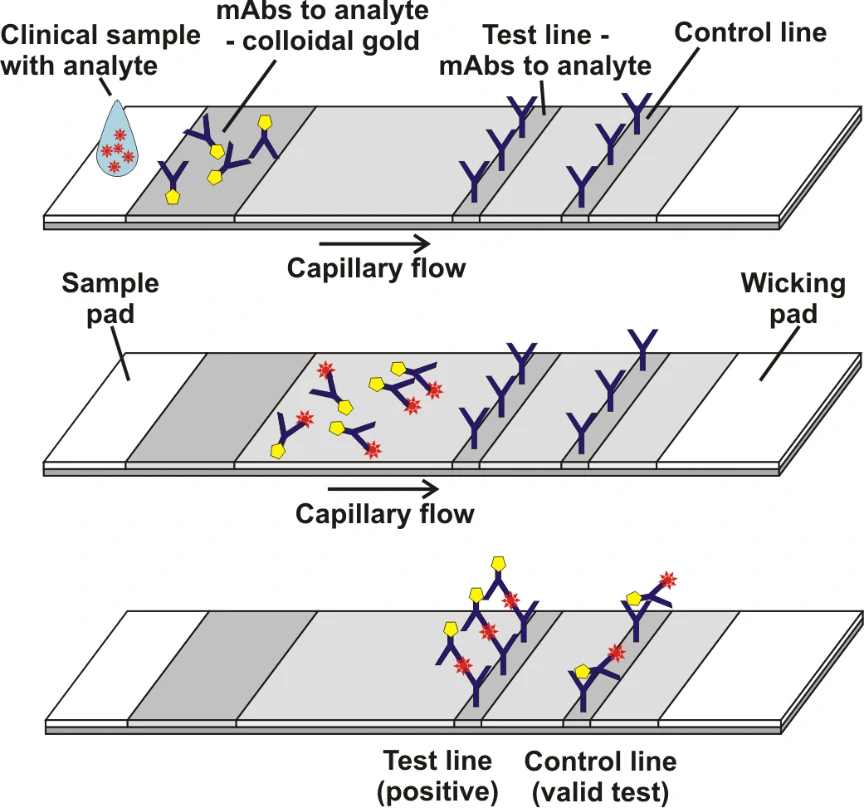
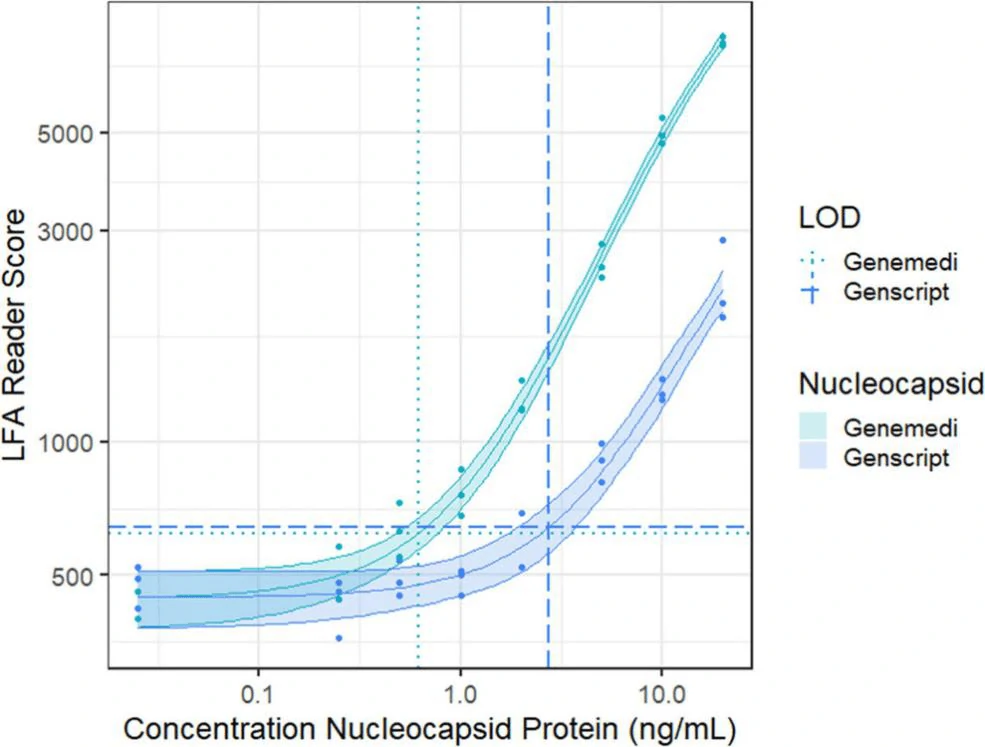
In this study, they presented a half-strip LFA for the detection of nucleocapsid protein of SARS-CoV-2. They used two commercially available SARS-CoV-2 nucleocapsid (N) proteins, from GeneMedi and Genscript to generate a dose response curve for the half-strip LFA. They found the limit of detection for the GeneMedi N protein was 0.65 ng/mL (95% CI of 0.53 to 0.77 ng/mL) and for the Genscript N protein was 3.03 ng/mL (95% CI of 0.00 to 7.44 ng/mL). The dose response curve, with 95% CI calculated in R using the drc package, is shown in Figure 2. The data indicated that the NP antigen from GeneMedi was a strong candidate for lateral flow assays (LFAs).
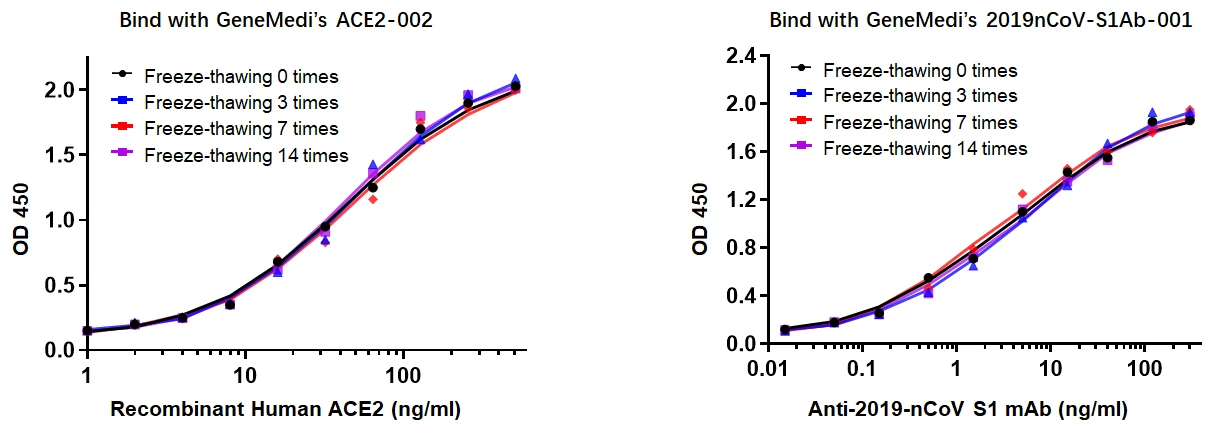
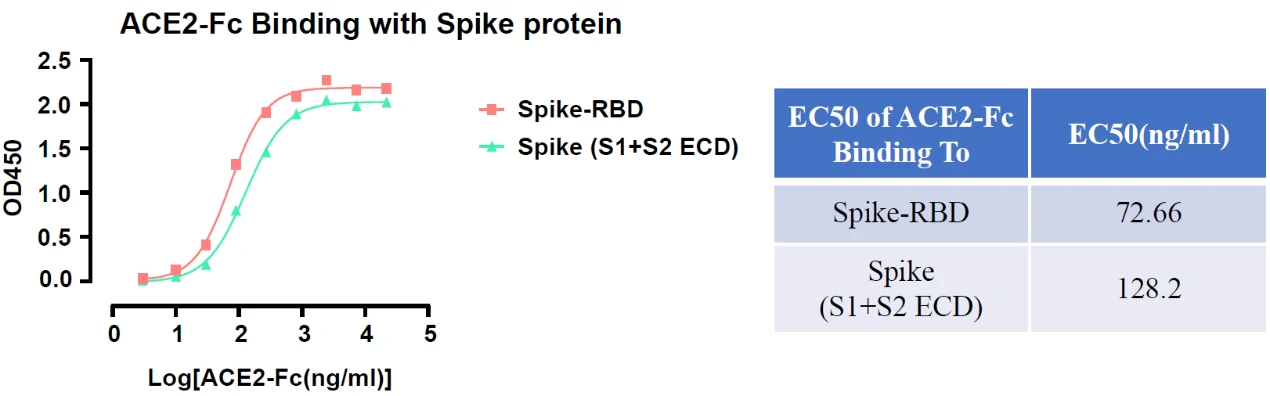
In addition to NP antigen, GeneMedi also developed and verified other recombinant SARS-CoV-2 antigens, they are applied by global diagnostics companies, and the customers have obtained good performances of these products, such as their stability and specificity. Take Spike RBD antigen (GMP-V-2019nCoV-SRBD001) as an example, below is the stability validation data of SRBD001. It shows that SRBD001 still exhibits excellent binding ability with ACE2002 and S1Ab001 after several freeze-thawing cycles.
In another study, Cate et al [3]. described an extensive antibody screening effort that utilized high-throughput robotic antibody screening platform to screen through 673 combinations of antibody pairs that target the SARSCoV-2 nucleocapsid protein. These anti-nucleocapsid antibody pairs were tested as both capture and detection reagents with the goal of finding those pairs that have the greatest affinity for unique epitopes of the nucleocapsid protein of SARS-CoV-2 while also performing optimally in an LFA format.
Biolayer interferometry was performed on recombinant nucleocapsid proteins (NPs), for the purpose of selecting the most “native-like” analyte for LFA antibody screening. Initially, they used the estimated Rmax of five different NPs to quantify binding affinity against a random selection of 21 anti-nucleocapsid antibodies from different vendors. The metric Rmax was calculated based on theoretically saturating 100% of the bound antibody (ligand) with the analyte (NP). The NP antigen from GeneMedi was selected as the starting antigen for antibody screening because the average saturation value across 21 different anti-NP antibodies was closest to the theoretical Rmax of the antigen.
Antibody pair screening on LFAs in first-round consisted of a 11 × 11 grid of antibodies (121 unique pairs). For each pair, one antibody was striped on nitrocellulose as a test line (the “capture” antibody) and the other was coupled to latex nanoparticles using EDC/NHS chemistry (the “detector” antibody). The results of the first-round antibody pair screening on LFAs are given in Figure 5.
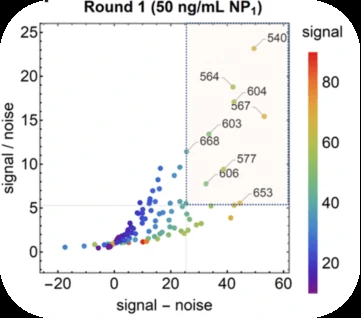
After the clinical based antibody pair screening, interestingly, they found the NP antigen from GeneMedi appeared to best predict antibody pair performance against clinical samples.
Antibody pairs for COVID-19 Serological Test and Immunoassay
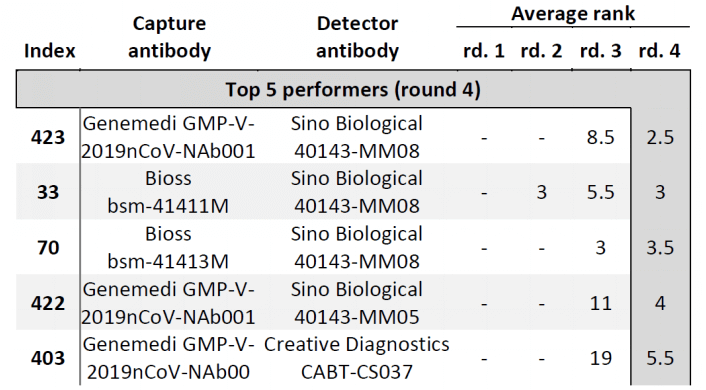
In Cate et al. ’s study[3], antibody screening results for anti-Nucleocapsid Antibodies towards the development of a SARS-CoV-2 Nucleocapsid Protein Antigen detecting Lateral Flow Assay, antibody pairs screening results in the top 5 in fourth-round from the clinical SARS-CoV-2 samples were shown in Figure 6. Among the top 5 antibody pairs, there were 3 antibody pairs from GeneMedi. This indicated that the NP antibodies from GeneMedi also behaved good performance in lateral flow assays (LFAs).
Besides these studies, GeneMedi also validated Spike antibodies and NP antibodies in pair test against COVID-19 in-house.
GeneMedi’s SARS-CoV2 NP antibodies were either in PBS solution (stocked in -20 for 7days) or lyophilized (stock at room temperature for 7 days). GeneMedi’s lyophilized antibodies presented excellent stability in the room temperature condition.
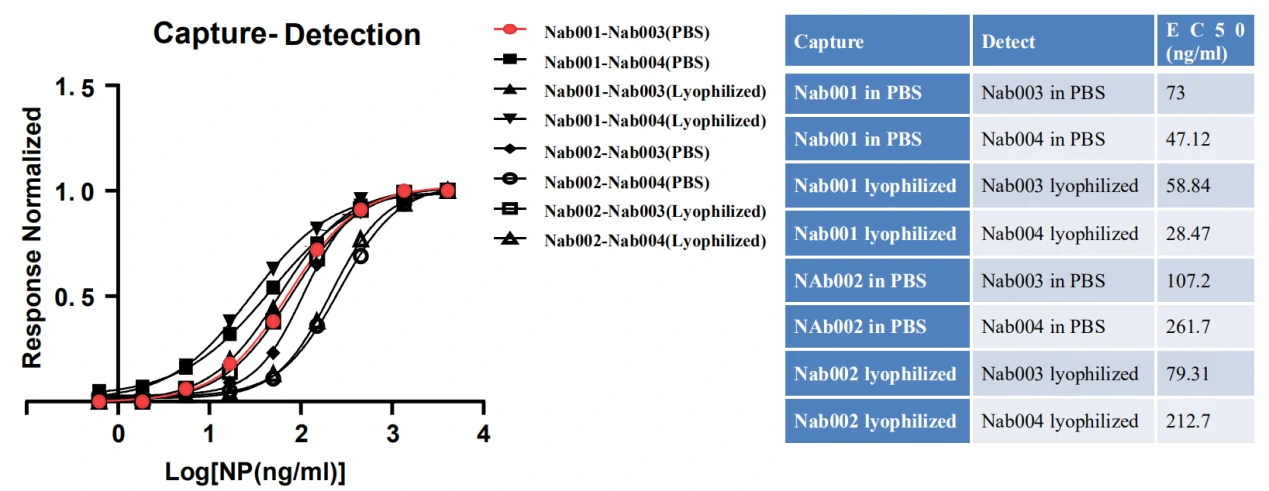
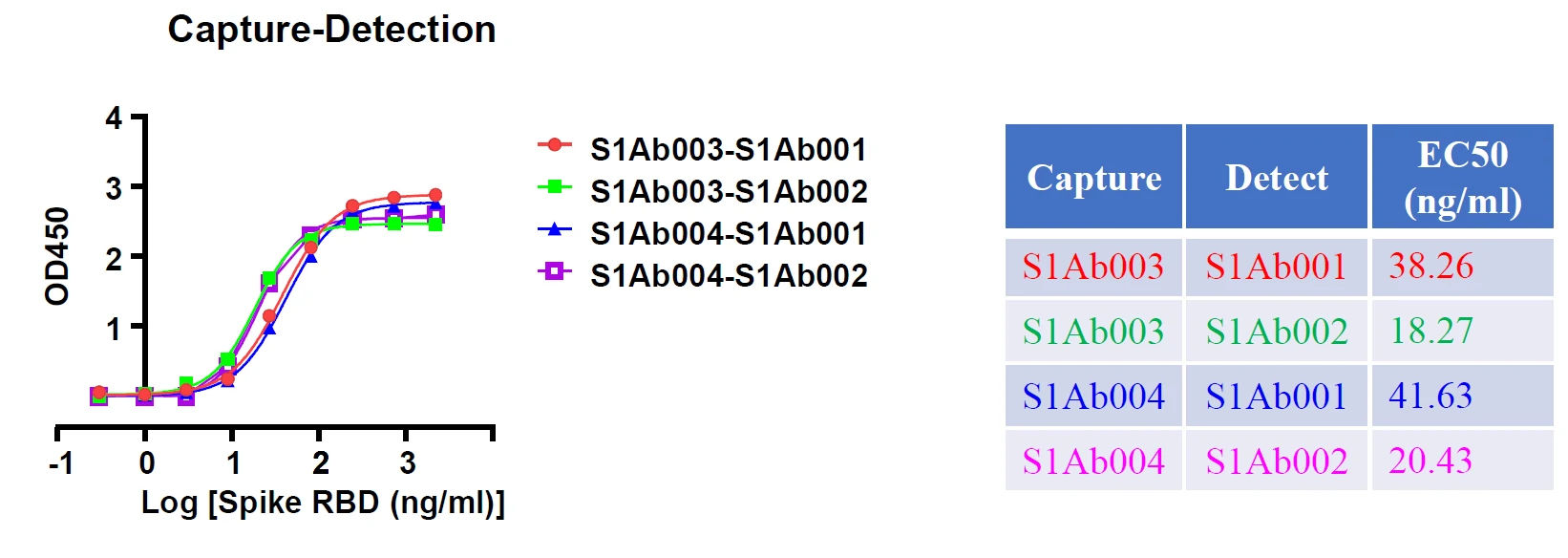
The data from these studies indicate that antigens and antibodies from GeneMedi show good performance as core ingredients of COVID-19 test kit.
References
DOI: 10.1021/acs.analchem.0c01975[2] https://med.unr.edu/ddl/technology/lateral-flow-immunoassay[3] Cate, David; Hsieh, Helen; Glukhova, Veronika; Bishop, Joshua D; Hermansky, H Gleda; Barrios-Lopez, Brianda; et al. (2020): Antibody Screening Results for Anti-Nucleocapsid Antibodies Towards the Development of a SARS-CoV-2 Nucleocapsid Protein Antigen Detecting Lateral Flow Assay. ChemRxiv. Preprint. https://doi.org/10.26434/chemrxiv.12709538.v1




