Diagnostic antibodies and antigens for functional ELISA assay kit, lateral flow and other immunoassays in diagnostics
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

Diagnostic antibodies and antigens list
In vitro diagnostic (IVD) testing has become an indispensable tool in clinical practice for diagnosing and monitoring of diseases, as well as providing prognosis and predicting treatment response, In vitro tests may be done in laboratories, health care facilities or even in the home. The tests themselves can be performed on a variety of instruments ranging from small, handheld tests to complex laboratory instruments. They allow doctors to diagnose patients effectively and work to provide appropriate treatments.
IVDs are used to analyze human samples such as blood and saliva, either by measuring the concentration of specific substances, or analytes (such as sodium and cholesterol), or by detecting the presence or absence of a particular marker or set of markers, such as a genetic mutation or an immune response to infection. Clinicians regularly use IVDs to diagnose conditions, guide treatment decisions, and even mitigate or prevent future disease (for example, through screening tests that indicate a patient’s risk of developing a given condition in the future). There are over 40,000 different IVD products available that provide information to doctors and patients on a huge range of conditions. These comprise markers for inorganic chemistry (electrolytes, toxins, and heavy metals), markers for organic chemistry/biochemistry (proteins, lipids, and carbohydrates), as well as molecular biologic procedures (sequencing and polymerase chain reaction).
In recent years, the value of IVDs has gained further recognition because of their potential to save lives through earlier diagnosis of medical conditions. In the case of an infectious disease, early detection can ensure that patients receive timely care to help slow or stop the progression of disease. Further, knowledge of infection can help promote self-isolation to prevent further spread of the disease to the community. This has been particularly important in containing the spread of highly infectious agents during an active outbreak. Using IVDs to predict underlying genetic conditions in addition to diagnostic screening can also prevent unnecessary suffering by a patient as well as reduce the scale of treatment required.
As the ease-of-use and reliability of IVDs has improved, some IVDs have moved out of the laboratory setting and into the patient’s home. Consumers can now perform simple tests to monitor blood glucose levels, identify urinary tract infections, track ovulation, and confirm pregnancy. These tests not only empower individuals to take charge of their own health but can provide quick results that help guide further medical actions. Genemedi provides diagnostic antibodies and antigens for the in vitro diagnosis of diseases such as infectious disease, inflammation/autoimmune/inflammatory disease, kidney function (renal damages), liver diseases, lung injury, metabolic diseases, multiple disease, neurodegenerative diseases, pain, thyroid diseases, vitamin deficiency and so on.
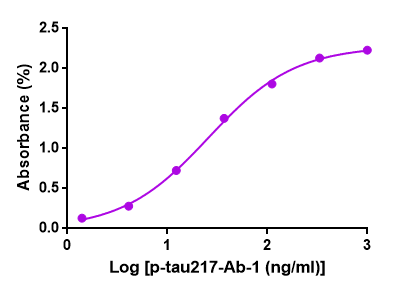
Evidence has shown plasma biomarkers (p-tau181, p-tau217, p-tau231, GFAP, NfL, and Aβ42/40) were significantly changed in preclinical Alzheimer’s Disease (AD). Recently, our R&D department demonstrated that our GMP-h-p-tau217-Ab01 has a large linear range and good sensitivity against the GMP-h-p-tau217-Ag01. Below is the result of GeneMedi’s GMP-h-p-tau217-Ab01 (Anti-human p-tau217 antibody) validation with GMP-h-p-tau217-Ag01 (p-tau217 antigen) in ELISA. We highly recommend the Ab&Ag to you.
Classification of IVD Disease:
Diagnostic antibodies and antigens for cancer detection - TG, CA-125, SCC Ag, PIVKA-Ⅱ, ProGRP, Calcitonin, NSE, CA-724, HE4, CA15-3, FER, CYFRA21-1, HER2, HSP90α, S100B, CEA, AFP, sFlt-1, PSA, hEGF, TFF2, TFF3, CA199, CA50, TK1
Regardless of the advances in the cancer therapy, delayed symptoms and lack of successful diagnosis of cancer at early stage increased the death rate. Tumor-associated antigens (TAAs) and their antibodies have been identified as potential markers in cancer diagnosis and determination. TAAs and their antibodies-based detection of cancer have the advantages such as low-cost, and simple access, which attracted much attention for early cancer detection. For several reasons, tumor marker itself is typically insufficient to analyze disease conditions. Because, maximum number of markers have been produced by both normal and cancer cells. Several cancers such as anal, breast, ovaries, testicles, colon, endometrial, peritoneal, fallopian tube, gallbladder, gastric, liver, lung, neuroendocrine, pancreatic, prostate, renal, cervix, stomach and thyroid cancer, bronchopulmonary dysplasia, hepatitis, hematological malignancies, hemochromatosis, hypercalcemia, osteoporosis, Parget’s diseases and so on have been identified using specific marker. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for infectious disease detection
It is important to diagnose the infectious disease even before it becomes serious. The traditional pathogen-detection methods, such as culture, have established their credibility over time, they are often slow and relatively insensitive. In addition, there are several emerging infectious diseases (ID) such as dengue fever, zika virus, corona virus and so on are need to be diagnosed immediately to prevent the outbreak. Immunodiagnostics show great promise than the traditional methods used in clinical diagnosis. GENEMEDI developed the antigens and antibodies for rapid kit such as competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody, and Lateral flow immunoassay (LFIA) ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT to detect the different types of infectious disease.
Diagnostic antibodies and antigens for Inflammation/autoimmune/inflammatory diseases testing-IL-6, PCT, CRP, SAA, IFNγ, AQP4, HBP, MOG, S100-A9, TPO, SAA1, TNFα, α defensin 5
Inflammation/autoimmune/inflammatory diseases occur when the immune system attacks the normal tissue within joints, vasculature, and other organ systems, causing inflammation, pain, diminished mobility, fatigue, and other non-specific symptoms. The strong overlap of signs and symptoms among the autoimmune diseases can lead to delays in diagnosis and appropriate treatment. However, immunodiagnostics has been developed to detect the inflammation/autoimmune/inflammatory disease such as ankylosing spondylitis, bacterial infections, bacterial meningitis, cerebrovascular diseases, crohn’s disease, fungal infection, graves’ disease, inflammatory amyloidosis, inflammatory bowel disease, mog antibody disease, osteomyelitis, rheumatoid arthritis, sepsis, respiratory illnesses and so on at an early stage. Biomarkers have been developed to detect this autoimmune disease at an early stage. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Cat No. | Species | Biomarker | Disease | Cat No.of Antigen | Bioactivity validation of Antigen | Cat No.of Antibodies | Bioactivity validation of Antibodies | Order |
|---|---|---|---|---|---|---|---|---|
Helicobacter pylori (H. pylori) |
Helicobacter pylori flagellin A |
GMP-IVD-P044-Tg002-Ag0101 |
Helicobacter pylori flagellin A (flagellin A (FlaA)) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in flagellin A (FlaA) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg002-Ab01/
GMP-IVD-P044-Tg002-Ab0201;GMP-IVD-P044-Tg002-Ab01/
GMP-IVD-P044-Tg002-Ab0202:Anti-Helicobacter pylori (H. pylori) flagellin A (FlaA) mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori flagellin A (flagellin A (FlaA)) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori flagellin A (flagellin A (FlaA)) antibodies in flagellin A (FlaA) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Helicobacter pylori (H. pylori) |
Helicobacter pylori flagellin B |
GMP-IVD-P044-Tg003-Ag0101 |
Helicobacter pylori flagellin B (flagellin B (FlaB)) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in flagellin B (FlaB) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg003-Ab01/
GMP-IVD-P044-Tg003-Ab0201;GMP-IVD-P044-Tg003-Ab01/
GMP-IVD-P044-Tg003-Ab0202:Anti-Helicobacter pylori (H. pylori) flagellin B (FlaB) mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori flagellin B (flagellin B (FlaB)) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori flagellin B (flagellin B (FlaB)) antibodies in flagellin B (FlaB) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Helicobacter pylori (H. pylori) |
Helicobacter pylori vacuolar cytotoxin A |
GMP-IVD-P044-Tg004-Ag0101 |
Helicobacter pylori vacuolar cytotoxin A (Vac A) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Vac A level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg004-Ab01/
GMP-IVD-P044-Tg004-Ab0201;GMP-IVD-P044-Tg004-Ab01/
GMP-IVD-P044-Tg004-Ab0202:Anti-Helicobacter pylori (H. pylori) Vac A mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori vacuolar cytotoxin A (Vac A) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori vacuolar cytotoxin A (Vac A) antibodies in Vac A level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Helicobacter pylori (H. pylori) |
Helicobacter pylori urease B |
GMP-IVD-P044-Tg005-Ag0101 |
Helicobacter pylori urease B (Ure B) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in Ure B level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg005-Ab01/
GMP-IVD-P044-Tg005-Ab0201;GMP-IVD-P044-Tg005-Ab01/
GMP-IVD-P044-Tg005-Ab0202:Anti-Helicobacter pylori (H. pylori) Ure B mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori urease B (Ure B) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori urease B (Ure B) antibodies in Ure B level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Helicobacter pylori (H. pylori) |
Helicobacter pylori heat shock protein |
GMP-IVD-P044-Tg006-Ag0101 |
Helicobacter pylori heat shock protein (HSP) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in HSP level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg006-Ab01/
GMP-IVD-P044-Tg006-Ab0201;GMP-IVD-P044-Tg006-Ab01/
GMP-IVD-P044-Tg006-Ab0202:Anti-Helicobacter pylori (H. pylori) HSP mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori heat shock protein (HSP) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori heat shock protein (HSP) antibodies in HSP level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Helicobacter pylori (H. pylori) |
Helicobacter pylori outer membrane protein 1 |
GMP-IVD-P044-Tg007-Ag0101 |
Helicobacter pylori outer membrane protein 1 (outer membrane protein 1 (OMP-1)) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in outer membrane protein 1 (OMP-1) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg007-Ab01/
GMP-IVD-P044-Tg007-Ab0201;GMP-IVD-P044-Tg007-Ab01/
GMP-IVD-P044-Tg007-Ab0202:Anti-Helicobacter pylori (H. pylori) outer membrane protein 1 (OMP-1) mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori outer membrane protein 1 (outer membrane protein 1 (OMP-1)) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori outer membrane protein 1 (outer membrane protein 1 (OMP-1)) antibodies in outer membrane protein 1 (OMP-1) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Helicobacter pylori (H. pylori) |
Helicobacter pylori outer membrane protein 3 |
GMP-IVD-P044-Tg008-Ag0101 |
Helicobacter pylori outer membrane protein 3 (outer membrane protein 2 (OMP-2)) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in outer membrane protein 2 (OMP-2) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation |
GMP-IVD-P044-Tg008-Ab01/
GMP-IVD-P044-Tg008-Ab0201;GMP-IVD-P044-Tg008-Ab01/
GMP-IVD-P044-Tg008-Ab0202:Anti-Helicobacter pylori (H. pylori) outer membrane protein 2 (OMP-2) mouse monoclonal antibody (mAb) |
Helicobacter pylori (H. pylori) Helicobacter pylori outer membrane protein 3 (outer membrane protein 2 (OMP-2)) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Helicobacter pylori outer membrane protein 3 (outer membrane protein 2 (OMP-2)) antibodies in outer membrane protein 2 (OMP-2) level test of Infectious disease (peptic ulcer? and gastritis) and related syndrome evaluation. |
|||
Prion |
Prion |
GMP-IVD-P045-Tg001-Ag0101 |
Prion (PrP) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in PrP level test of Infectious disease (Transmissible spongiform
encephalopathies) and related syndrome evaluation |
GMP-IVD-P045-Tg001-Ab01/
GMP-IVD-P045-Tg001-Ab0201;GMP-IVD-P045-Tg001-Ab01/
GMP-IVD-P045-Tg001-Ab0202:Anti-Prion PrP mouse monoclonal antibody (mAb) |
Prion Prion (PrP) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Prion (PrP) antibodies in PrP level test of Infectious disease (Transmissible spongiform
encephalopathies) and related syndrome evaluation. |
|||
Sexually transmitted diseases |
GMP-IVD-P046-Tg001-Ag0101 |
Sexually transmitted diseases (STDs) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in STDs level test of Infectious disease (Sexually transmitted diseases) and related syndrome evaluation |
GMP-IVD-P046-Tg001-Ab01/
GMP-IVD-P046-Tg001-Ab0201;GMP-IVD-P046-Tg001-Ab01/
GMP-IVD-P046-Tg001-Ab0202:Anti- STDs mouse monoclonal antibody (mAb) |
Sexually transmitted diseases (STDs) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other Sexually transmitted diseases (STDs) antibodies in STDs level test of Infectious disease (Sexually transmitted diseases) and related syndrome evaluation. |
||||
acute respiratory infectious diseases |
GMP-IVD-P047-Tg001-Ag0101 |
acute respiratory infectious diseases (ARTI) antibodies binding, Immunogen in Sandwich Elisa, lateral-flow tests, and other immunoassays as control material in ARTI level test of Infectious disease (acute respiratory infectious diseases) and related syndrome evaluation |
GMP-IVD-P047-Tg001-Ab01/
GMP-IVD-P047-Tg001-Ab0201;GMP-IVD-P047-Tg001-Ab01/
GMP-IVD-P047-Tg001-Ab0202:Anti- ARTI mouse monoclonal antibody (mAb) |
acute respiratory infectious diseases (ARTI) antigen binding, ELISA validated as capture antibody and detection antibody. Pair recommendation with other acute respiratory infectious diseases (ARTI) antibodies in ARTI level test of Infectious disease (acute respiratory infectious diseases) and related syndrome evaluation. |
Diagnostic antibodies and antigens for cardiovascular diseases biomarkers testing
Cardiovascular diseases are a group of disorders that occur in the heart and blood vessels and are the world’s leading cause of death. In vitro diagnostic (IVD) assays for detecting cardiac markers are essential tools to help treat or prevent CVD. Several protein markers are released into the blood when the heart is damaged or stressed. These protein markers support diagnosis of conditions including coronary heart disease, cerebrovascular disease, and deep vein thrombosis to guide therapeutic intervention. Several cardiovascular diseases such as hypertension, coronary artery stenosis, intrinsic cardiac dysfunction, acute ischemic myocardial injury, acute myocardial infarction, angiogenesis, artery lesions, atherosclerosis, blood pressure, congestive heart failure, Deep vein thrombosis (DVT), heart failure, hypertrophic cardiomyopathy, myocardial infarction, renal artery stenosis, restrictive and dilated cardiomyopathy, vascular inflammation and so on have been identified using specific marker. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for Liver Diseases testing: Hyaluronic acid (HA), laminins (LN), collagen type IV (cIV), Procollagen III N-terminal peptide (PIIINP), Lithocholic Acid (LCA), deoxycholic acid (cholanoic acid), hyaluronic acid (HA), Cholyglycine (CG), Prealbumin (PA)
Liver is an important organ and it is responsible for the metabolism, energy storage, and waste filtering. Liver disease is a generally designates to any condition that affects the liver. These conditions may be because of different reasons, but all these reasons damage the liver and affect its function. Many people with liver disease do not cause any symptoms in the early stages. At a certain point in the development of liver disease damage can become irreversible and lead to liver failure, liver cancer, or death. Early diagnosis of liver disease may prevent liver damage. GENEMEDI produces core diagnostic ingredients such as collagen type IV, Procollagen III N-terminal peptide, Lithocholic Acid, deoxycholic acid, hyaluronic acid, Cholyglycine, Prealbumin and so on for test of Liver Diseases and related syndrome using ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT Kit.
Diagnostic antibodies and antigens for lung diseases testing: ACE2/hFc, ACE2/his
Lung disease such as asthma, COPD, infections like influenza, pneumonia and tuberculosis and lung cancer can affect respiratory function, or the ability to breathe, and pulmonary function. some of the lung diseases are caused by bacterial, viral, or fungal infections and some other lung diseases are associated with environmental factors, including asthma, mesothelioma, and lung cancer. The early diagnosis of the lung disease is an important step to prevent the respiratory failure. Biomarker such as human angiotensin converting enzyme 2 and so on are suitable for monitoring lung disease at early stages. Detecting the quantity of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for kidney function (renal damages) testing: Beta-2 microglobulin (B2M), Cystatin C (CYSC), Neutrophil gelatinase-associated lipocalin (NGAL), Alpha-1-microglobulin (A1M)
The kidneys are important to filter wastes and excess fluids from the blood and excreted in urine. Chronic kidney disease steadily loss the kidney function and fail to filter the waste in the body. The chronic kidney disease (CKD) includes advanced stage of renal parenchyma damage and the loss of functional nephrons. The loss of functional nephrons induces several molecular and cellular events, which leads to the development of renal lesions. The chronic kidney disease disperses the podocytes from basal membrane and excrete in the urine, therefore the diagnosis usually made with the serum proteins (creatinine) and blood urea (sCr). The early diagnosis of this disease is an important step to prevent the CKD. Biomarker such as Cystatin C (CYSC) Neutrophil gelatinase-associated lipocalin (NGAL),Beta-2 microglobulin (B2M),Alpha-1-microglobulin (A1M) and so on are suitable for monitoring CKD at early stages. Detecting the quantity of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for gastric disease testing: pepsinogen I (PG I), Pepsinogen II (PG II), Gastrin 17 (G17), trefoil factor 1 (TFF1), Gastrin (G100)
Gastric disorders are the most common disease leads to the death. Most death of gastric disease is mostly due the diagnosis at late-stage. The diagnosis of Gastric disease at early-stage is delayed due to the lack symptoms and effective diagnostic technique. The traditional technology, including endoscopic examination and gastroscopic biopsy are invasive to screen large population. Hence, biomarkers such as pepsinogen I, Pepsinogen II, Gastrin 17, trefoil factor 1, Gastrin and so on have been developed to detect the gastric diseases at an early stage for large population to significantly reduce the death caused by gastric cancer. However, the detection of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for thyroid disease testing: Reverse triiodothyronine (rT3), Thyroid Stimulating Hormone (TSH)
Thyroid gland produces the hormones that keep body functioning normally. When concentration of these hormone increases or decrease, it’s called a thyroid disease. There are several types of thyroid disease, which includes hyperthyroidism, hypothyroidism, thyroiditis and Hashimoto’s thyroiditis. The most commonly measured thyroid hormones are thyroid stimulating hormone (TSH), total thyroxine (T4), free T4, total triiodothyronine (T3), and free T3. The thyroid gland produces T4, as well as some T3. After its release from the thyroid gland, T4 is converted to T3, which is an active thyroid hormone, or to Reverse triiodothyronine (rT3), which is considered an inactive form. The rate and ratio of T4 conversion to either T3 or rT3 is depends upon the body’s metabolic requirement. Hence, the measurement of reverse triiodothyronine, thyroid-stimulating hormone and so on is useful to diagnose the thyroid disorder. GeneMedi offers diagnostic antigen and antibody to detect the thyroid disorder.
Diagnostic antibodies and antigens for inflammatory bowel disease testing: Calprotectin (S100-A8)
Inflammatory bowel disease (IBD) is a group of idiopathic inflammatory disorders of the gastrointestinal (GI) tract characterized by the symptoms of abdominal pain and diarrhea. IBD is diagnosed by the comprehensive analysis of clinical findings, radiological imaging, invasive endoscopy and histopathological examination. Due to a lack of a gold standard, certain patients do not receive a definitive diagnosis using current diagnostic criteria. Recently, the search for a noninvasive marker that could augment or replace part of this diagnostic process has become a focus of IBD research. antibody markers, including microbial antibodies, autoantibodies and peptide antibodies, will minimize the use of endoscopic and radiologic examinations and improve the long-term prognosis of patients with IBD. Several intestinal inflammations and so on have been identified using specific marker. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for fertility testing: Anti-Mullerian hormone (AMH), Fetal fibronectin (fFN), acrosome protein (ACRV1)
The pregnancy contains multiple steps that must effectively happen at a precise time and in the precise place. For fertilization, oocytes and sperm cells must experience a series of differentiation and development process. Abnormalities on the placement of embryo in the woman’s uterus, decidualization, placentation and intrauterine embryonic development consequence the onset of pre-eclampsia, miscarriage and/or preterm birth. On the other hand, sperm cells of the man should undergo capacitation and acrosome exocytosis to fertilize oocytes appropriately. A little imbalance in this process may disturb the capability of sperm cells to fertilize the egg. Thus, the detection of biomarkers to monitor the pregnancy process, and complication in both female and male gametes is an opportunity for successful pregnancy. Recently, several biomarkers have been developed to improve diagnostic accuracy. Indeed, GENEMEDI offer the biomarkers such as Anti-Mullerian hormone (AMH), Fetal fibronectin (fFN), acrosome protein and so on to make a successful pregnancy. Detecting the quantity of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for diabetes testing: C-peptide, Copeptin, Human Insulin (Ins), Human Proinsulin (Proinsulin), Adiponectin (ADPN)
Diabetes mellitus is the most known chronic disorder characterized by hyperglycemia. Diabetes mellitus is classified based on the deficiency of insulin production due to β-cell destruction (type 1) and by increased insulin resistance (type 2). The increasing rate of obesity increased the difficulties to determine the type of diabetes mellitus. The classification of diabetes mellitus is important for treatment. Beside the conventional method, the detection of biomarkers such as c-peptide, copeptin, insulin, proinsulin, adiponectin and so on are used to differentiate the diabetes mellitus type. However, the detection of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for metabolic diseases testing: 25-OH-(VD3+VD2), PINP, β-CTx, 25-OH-VD, 25-OH-VD-3, SAHH, CBL, CBS, LP-PLA2, RBP4
Metabolism is the process of making energy from the food. metabolic disease, any of the diseases or disorders that disrupt normal metabolism, the process of converting food to energy on a cellular level. Thousands of enzymes participating in numerous interdependent metabolic pathways carry out this process. Metabolic diseases affect the ability of the cell to perform critical biochemical reactions that involve the processing or transport of proteins (amino acids), carbohydrates (sugars and starches), or lipids (fatty acids). The consequences of metabolic disorder may be severe; intellectual disability, seizures, decreased muscle tone, organ failure, blindness, and deafness may occur, depending on which enzyme is dysfunctional. Biomarker such as -25 hydroxyvitamin D, amino-terminal propeptide (PINP), β-isomerized C-terminal telopeptides, 25-hydroxy (OH) Vitamin, S-adenosylhomocysteine (AdoHcy) hydrolase, cystathionine-β-synthase enzyme, Phospholipase A2, Retinol binding protein 4 and so on are suitable for monitoring metabolic disease. Detecting the quantity of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for hormonal disorders testing: DHEA, DEX, Calcitonin, Cortisol, Testosterone, PG, EPO, Progesterone, LH, Prolactin, Estrone, Estradiol, HCG, DES/HES, Bisphenol A, Thyroxin T4, Forchlorfenuron, Norethisterone, Flugestone Acetate, Thyroxin T3, IGFBP-1, ACTH, PTH, TBG, FSH
Hormones are proteins, which help organs and tissues to perform specific functions. hormones play an integral role in your overall health. It controls growth, fertility, sexual function, emotions, and absorption of nutrients. Hormone disorders are the result of a hormonal imbalance i.e production of too much or too little of one or more hormones. Several hormonal disorders such as Anemia, hypothyroidism, irregular bleeding, pituitary or adrenal malfunction, acromegaly, acute inflammation, breast cancer, Cushing’s syndrome, erythrocytosis, gigantism, hypergonadotrophic-hypogonadism, Hyperparathyroidism, hypertension, hypopituitarism, infertility, mood swings, polycythemia, pregnancy, Testosterone Deficiency Syndrome, thyroid cancer and so on have been identified using specific marker. Detecting the quantity of marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for Vitamin deficiency testing: folic acid (FA), vitamin D binding protein (VDBP)
Vitamin deficiency is the condition of a long-term lack of a vitamin. Vitamin deficiency caused by insufficient intake of vitamin is classified as a primary deficiency, whereas vitamin deficiency due to an underlying disorder such as malabsorption is called as a secondary deficiency. Vitamin deficiencies can be detected in several ways. GENEMEDI produces core diagnostic ingredients such as folic acid, vitamin D binding protein and so on to test vitamin deficiency using ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT Kit.
Diagnostic antibodies and antigens for Fibrinogen disorders testing: Fibrinogen (FIB)
Fibrinogen disorders are a set of hereditary or acquired abnormalities in the quantity and/or quality of circulating fibrinogens. The disorders may lead to pathological bleeding and/or blood clotting or the deposition of fibrinogen in the liver, kidneys, or other organs and tissues. These disorders include Congenital afibrinogenemia, Congenital hypofibrinogenemia, Fibrinogen storage disease, Congenital dysfibrinogenemia, Hereditary fibrinogen Aα-Chain amyloidosis, Acquired dysfibrinogenemia, Congenital hypo dysfibrinogenemia and Cryofibrinogenemia. The detection of fibrinogen and so on are used to diagnose the fibrinogen disorders. However, the detection of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for Neurodegenerative diseases testing: Phospho-tau181 (p-tau181), Phospho-tau217 (p-tau217), tau proteins (Tau), neurofilament light chain (NFL), neurofilament protein (AD7c-NTP)
Neurodegenerative diseases refer to the injury or dysfunction of one or more nerves, which results in numbness, tingling, muscle weakness and discomfort in the nerves affected part. Neurodegenerative diseases often arise from the hands and feet, sometimes other parts of the body also affected. Peripheral neuropathy refers the injury or dysfunction of the peripheral nerves. Peripheral neuropathy might be acute or chronic, and sometimes it is reversible. Neurodegenerative diseases induce a series of complications in the health and develop the symptoms of those issues. Biochemical markers are increasingly used for the early diagnosis of the neuropathy. In addition, such markers may provide additional information for understanding the pathology of disease. Several markers such as a Phospho-tau181, Phospho-tau217, tau proteins, neurofilament light chain, neurofilament protein and so on have been identified to be associated with the neurodegenerative diseases. Detecting the quantity of these marker proteins from different samples may benefit from ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for Epilepsy-related valproate (VPA) toxicity testing: Sodium valproate (VPA)
Valproate is a commonly used antiepileptic drug for the treatment of epilepsy and seizures. It is safe for use in both adults and children more than three years of age. Valproate toxicity can occur both accidentally and intentionally. Acute valproate overdose usually presents with CNS depression/encephalopathy, electrolyte abnormalities such as hypernatremia, elevated transaminase levels, hyperammonemia, and hepatoxicity. In patients with a severe overdose of valproate, patients can present with hypotension, tachycardia, respiratory depression, metabolic acidosis, cerebral edema, and valproate-related hyperammonemic encephalopathy which may progress to coma and death. GENEMEDI developed the Gastrin-17 (G-17) antigens and antibodies for the diagnosis of valproate toxicity based on ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for pain-related acetaminophen (Paracetamol) toxicity testing
Acetaminophen is known as paracetamol in many countries outside the US. It is one of the most frequently used oral analgesics and antipyretics. It has an outstanding safety profile when administered in an appropriate dose, but hepatotoxicity can occur after overdose or when misused in at-risk populations. GENEMEDI developed the antigens and antibodies for the diagnosis of potential acetaminophen toxicity based on ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT.
Diagnostic antibodies and antigens for multiple disease testing: thioredoxin reductase (TrxR)
Thioredoxin (Trx) and thioredoxin reductase (TrxR) plus NADPH, comprising the thioredoxin system, has a large number of functions in DNA synthesis, defense against oxidative stress and apoptosis or redox signaling with reference to many diseases such as cancer, viral disease, ischaemia– reperfusion injury, cardiac conditions, aging, premature birth and newborn physiology. GENEMEDI produces core diagnostic ingredients such as thioredoxin reductase and so on to test multiple disorders using ELISA, Lateral flow immunoassay (LFIA), colloidal gold immunochromatographic assay, Chemiluminescent immunoassay (CLIA), turbidimetric inhibition immuno assay (TINIA), immunonephelometry and POCT Kit.
Diagnostic antibodies and antigens for biologics testing: Streptavidin (SA), Staphylococcus protein A (SPA)
The biological agent such as streptavidin and staphylococcus protein A have an important role in biochemical research because of its ability to bind biotin and immunoglobulins respectively. GENEMEDI produces core diagnostic ingredients such as streptavidin, staphylococcus protein A and so on for the biochemical research.




