Endogenous coupling of amino acids and Disulfide re bridging strategy
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

Endogenous coupling of amino acids
One of the most common coupling methods is to use the lysine residue of the antibody, the amino acid nucleophilic NH2 group, to react with the electrophilic N-hydroxysuccinimide (NHS) Group on the lik payload. Although the reaction is simple, the high abundance of available lysine residues leads to the formation of uneven mixtures of many ADCs under random distribution. DAR is controlled by antibody-drug conjugates stoichiometry, which is widely used, including approved ADCs such as Besponsa, Mylotarg, and Kadcyla.
Disulfide re bridging strategy
IgG antibodies contain four disulfide bonds between chains, two connecting light chains and heavy chains, and two are located in the hinge region connecting two heavy chains. They maintain the integrity of monoclonal antibodies. Another classic biological coupling pathway explores the role of these cysteines as payload attachment points. The reduction of four disulfide bonds usually produces eight sulfhydryl groups, which can react with the linker of maleimide to produce ADC with DAR=8.
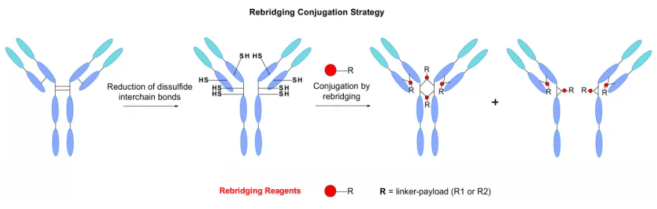
Dorona and colleagues reported an example of an ADC with a chimeric anti-CD30 monoclonal antibody coupled to MMAE, DAR= 8. Compared with the classical lysine coupling, this payload loading method is better controlled. However, it is reported that the plasma clearance rate will be higher and the risk of plasma aggregation will be reduced.
In 2015, chudasama et al. Introduced a new type of re bridging reagent, dibromopyridazinediones. They proved that it can be effectively inserted into the disulfide bond, and the resulting structure shows excellent hydrolytic stability even at high temperature. However, with the increase of temperature on the reduction step, heterogeneity is also observed, and this structure also allows the selective introduction of different functional groups.
Divinylpyrimidine is another effective re-bridging reagent, which can produce stable ADC with Dar = 4. Spring et al. Studied the effect of vinyl heteroaryl scaffold on cysteine re bridging. They believe that replacing pyridine with pyrimidine can make heteroaryl ring a better electron acceptor, so as to improve the crosslinking efficiency. Their work extends to divinyltriazine, which shows higher efficiency at high temperature.
In order to avoid the disadvantage of in vivo instability associated with classical maleimide coupling, Barbas et al studied methylsulfonylphenyloxadiazole, which has a specific reaction to cysteine. They are more stable than cysteine maleimide conjugates in plasma. Inspired by this, Zeglis designed dipods reagent, which contains two oxadiazole methyl sulfone parts connected by phenyl. Dipods forms covalent bonds with two sulfate radicals in the way of re bridging. Compared with maleimide coupling, coupling in this way has superior stability in vitro and performance in vivo.
Product List
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Losatuxizumab Vedotin Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
losatuxizumab vedotin |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lupartumab Amadotin Biosimilar, Whole Mab Adc, Anti-Lypd3 Antibody: Anti-C4.4A therapeutic antibody Drug Conjugate |
lupartumab amadotin |
NA |
LYPD3 |
Whole mAb ADC |
||
Pre-Made Mipasetamab Uzoptirine Biosimilar, Whole Mab Adc, Anti-Axl Antibody: Anti-ARK/JTK11/Tyro7/UFO therapeutic antibody Drug Conjugate |
mipasetamab uzoptirine |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Mirvetuximab Soravtansine Biosimilar, Whole Mab Adc, Anti-Folr1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody Drug Conjugate |
mirvetuximab soravtansine |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Mirzotamab Clezutoclax Biosimilar, Whole Mab Adc, Anti-Cd276 Antibody: Anti-4Ig-B7-H3/B7-H3/B7H3/B7RP-2 therapeutic antibody Drug Conjugate |
mirzotamab clezutoclax |
NA |
CD276 |
Whole mAb ADC |
||
Pre-Made Naratuximab Emtansine Biosimilar, Whole Mab Adc, Anti-Cd37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody Drug Conjugate |
naratuximab emtansine |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Patritumab Deruxtecan Biosimilar, Whole Mab Adc, Anti-ERBB3/Erbb-3 Antibody: Anti-ErbB-3/FERLK/HER3/LCCS2/MDA-BF-1/VSCN1/c-erbB-3/c-erbB3/erbB3-S/p180-ErbB3/p45-sErbB3/p85-sErbB3 therapeutic |
patritumab deruxtecan |
NA |
ERBB3 |
Whole mAb ADC |
||
Pre-Made Pelgifatamab Corixetan Biosimilar, Whole Mab Adc, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody Drug Conjugate |
pelgifatamab corixetan |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Pinatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd22 Antibody: Anti-SIGLEC-2/SIGLEC2 therapeutic antibody Drug Conjugate |
pinatuzumab vedotin |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Pivekimab Sunirine Biosimilar, Whole Mab Adc, Anti-Il3Ra Antibody: Anti-CD123/IL3R/IL3RX/IL3RY/hIL-3Ra therapeutic antibody Drug Conjugate |
pivekimab sunirine |
NA |
IL3RA |
Whole mAb ADC |
||
Pre-Made Polatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody Drug Conjugate |
polatuzumab vedotin |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Praluzatamab Ravtansine Biosimilar, Whole Mab Adc, Anti-Alcam Antibody: Anti-CD166/MEMD therapeutic antibody Drug Conjugate |
praluzatamab ravtansine |
NA |
ALCAM |
Whole mAb ADC |
||
Pre-Made Rolinsatamab Talirine Biosimilar, Whole Mab Adc, Anti-Prlr Antibody: Anti-HPRL/MFAB/RI-PRLR/hPRLrI therapeutic antibody Drug Conjugate |
rolinsatamab talirine |
NA |
PRLR |
Whole mAb ADC |
||
Pre-Made Rosopatamab Tetraxetan Biosimilar, Whole Mab Adc, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody Drug Conjugate |
rosopatamab tetraxetan |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Rovalpituzumab Tesirine Biosimilar, Whole Mab Adc, Anti-Dll3 Antibody: Anti-SCDO1 therapeutic antibody Drug Conjugate |
rovalpituzumab tesirine |
NA |
DLL3 |
Whole mAb ADC |
||
Pre-Made Sacituzumab Govitecan Biosimilar, Whole Mab Adc, Anti-Tacstd2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody Drug Conjugate |
sacituzumab govitecan |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Samrotamab Vedotin Biosimilar, Whole Mab Adc, Anti-Lrrc15 Antibody: Anti-LIB therapeutic antibody Drug Conjugate |
samrotamab vedotin |
NA |
LRRC15 |
Whole mAb ADC |
||
Pre-Made Serclutamab Talirine Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
serclutamab talirine |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Sirtratumab Vedotin Biosimilar, Whole Mab Adc, Anti-Slitrk6 Antibody: Anti-DFNMYP therapeutic antibody Drug Conjugate |
sirtratumab vedotin |
NA |
SLITRK6 |
Whole mAb ADC |
||
Pre-Made Sofituzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Muc16 Antibody: Anti-CA125 therapeutic antibody Drug Conjugate |
sofituzumab vedotin |
NA |
MUC16 |
Whole mAb ADC |
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




