Immunoassay in Coronavirus Disease 2019 (COVID-19) test
Diagnostic antibodies and antigens for Companion Animal disease testing
● Rabbit
Diagnostic antibodies and antigens for Swine disease testing
Diagnostic antibodies and antigens for Avian disease testing
Diagnostic antibodies and antigens for Multiple animal disease testing
Diagnostic antibodies and antigens for Ruminant disease testing
● Deer
Diagnostic antibodies and antigens for infectious and non-infectious Equine/Horse disease testing
SOCAIL MEDIA

Author:
Huajun Bai1 Xiaolong Cai1, 2* Xiaoyan Zhang1
1. R&D Center, GeneMedi Co.Ltd., Shanghai, P.R. China (www.genemedi.com)
2. Hanbio Research Center, Hanbio Tech Co. Ltd., Shanghai, P.R. China (www.hanbio.net)
Abstract:
The outbreak of COVID-19, caused by 2019 novel coronavirus (2019-nCoV), has been a global public health threat and caught the worldwide concern. Scientists throughout the world are sparing all efforts to explore strategies for the determination of the 2019-nCoV virus and diagnosis of COVID-19 rapidly. Several assays are developed for COVID-19 test , including RT-PCR, coronavirus antigens-based immunoassays, and CRISPR-based strategies (Cas13a or Cas12a), etc. Different assays have their advantages and drawbacks, and people should choose the most suitable assay according to their demands. Here, we make a brief introduction about these assays and give a simple overview of them, hoping to help doctors and researchers to select the most suitable assay for the Coronavirus Disease 2019 test (COVID-19 test) .
Immunoassay in Coronavirus Disease 2019 (COVID-19) test
1) Point-of-care lateral flow test
A. Principles for Diagnostics
Once entry into host cells, SARS-CoV-2 will provoke the adaptive immune system in the body of the host. 3-6 days post-infection, IgM antibody will be produced, while IgG antibody will be generated 8 days post-infection. Thus, these antibodies against SARS-CoV-2 can be detected in patient’s blood. Moreover, IgM tends to be an indicator of the recent exposure of virus, while IgG indicates earlier virus infection. The IgM and IgG can be determined based on highly specific antibody-antigen interactions. In brief, add 10-15μl blood specimens and 70μl sample dilution buffer to the sample port. When the blood specimen flows through the SARS-CoV-2 recombinant antigen (receptor binding domain of SARS-CoV-2 spike protein) labeled by 40nm gold nanoparticle (AuNP) colloids via chromatographic lateral flow, a complex of IgM-antigen-AuNP/IgG-antigen-AuNP will form. Then this complex continues to move on and passes through the coated mouse anti-human IgM/G antibody at M/G coating line, they will form a double antibody sandwich colloidal gold complex, showing a color band at the coating line (red/purple). The excessive fluid continues to flow through the Control line, Rabbit IgG conjugated AuNP will interact with Goat anti-Rabbit IgG antibody, exhibiting a color band at the control line [8]. This point-of-care lateral flow test utilizes a colloidal gold as an indicator to determine the IgM/G against SARS-CoV-2, which is a reliable and visual index for the rapid screening of SARS-CoV-2 carriers (Fig. 3).
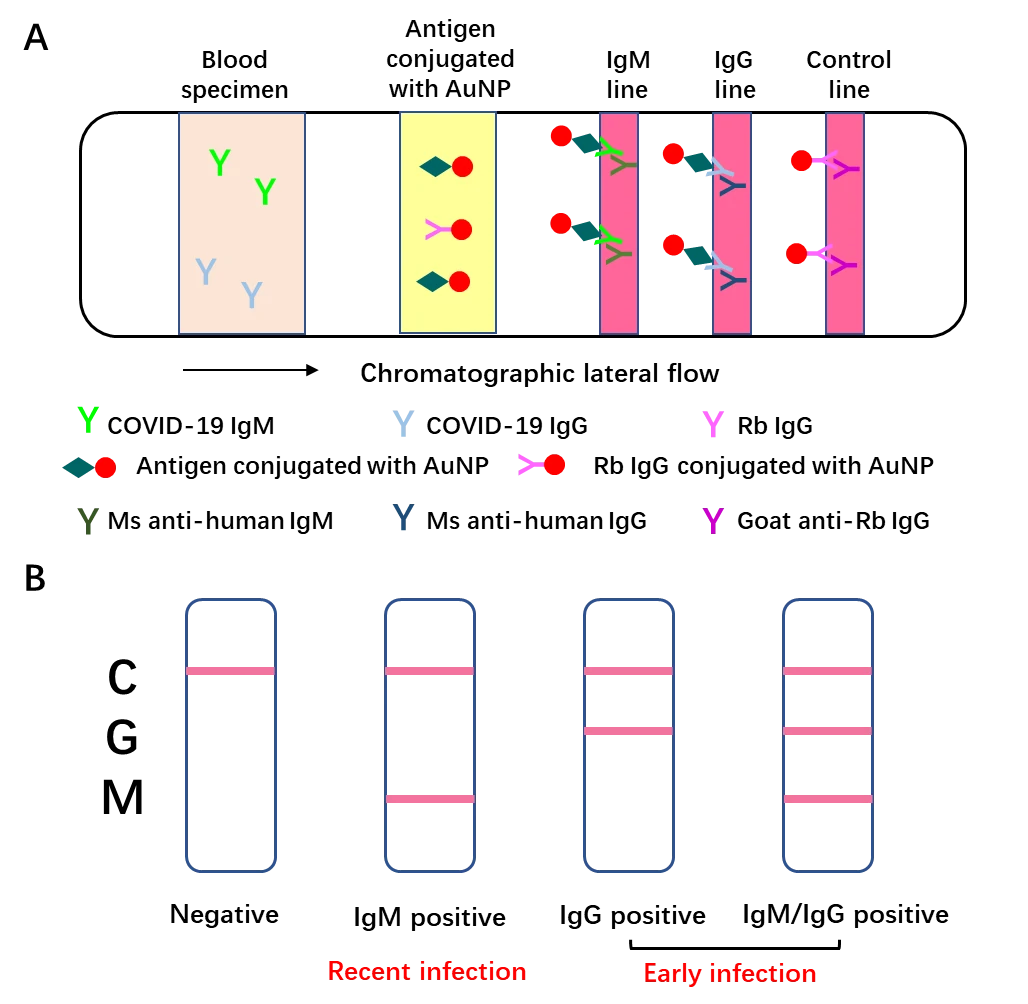
B. Advantages and disadvantages
Advantages:
① Easy to use and operate on a large scale.
② No requirement for additional equipment.
③ High sensitivity (88.66%) and specificity (90.63%).
④ Cost little time, less than 15 minutes.
Disadvantages:
Long window period. It’s difficult to detect the early infections, for IgM antibody could only be detected in patient’s blood 3-6 days post-infection, while IgG can be detected 8 days post-infection.
C. Optional Targets
The targets of point-of-care lateral flow tests are human antibodies against SARS-CoV-2 antigens. SARS-CoV-2 mainly has four structural proteins with great genetic similarity to those of SARS-CoV, including S (76.0%), E (94.7%), M (90.1%), and N (90.6%). Based on the antigen epitope analysis of SARS-CoV and SARS-CoV-2 [9], N protein and S protein are expected to be potential antigen targets with a large number of epitopes for the induction of both T cell response and B cell response, which might show promising prospects for vaccine development and induction of long-term immune responses. Additionally, several structural shreds of evidence show S protein is crucial for the entry of SARS-CoV-2 into host cells [2-4, 10] and administration of antibody against SARS-CoV S protein blocks SARS-CoV-2 S mediated entry into cells, which might give a blueprint for COVID-19 vaccine development targeting S protein epitopes [3].
2) ELISA and the other immunoassay in Coronavirus Disease 2019 (COVID-19) test
Besides the above Point-of-care lateral flow test, ELlSA also can be utilized to quantify SARS-CoV-2 antigens via the double antibody sandwich method. Briefly, coat the plate with capture antibody, which can interact with antigens and pull the antigens down once sample is added. Then detection antibody, conjugated with biotin or fluorophores, is added to bind to the other epitope of antigens. Thus, antigen can be detected with capture antibody and detection antibody and labeled by biotin or fluorophores. Additionally, when SARS-CoV-2 infects humans, the immune system will generate a pile of cytokines, including IL2, IL7, IL10, GSCF, IP10, MCP1, MIP1A, and TNFα [11]. These cytokines can also be detected by ELISA or other immunoassays, such as Western blot or immunostaining.
3) Core for COVID-19 immunoassay: recombinant 2019nCoV antigens
All of these immunoassays for COVID-19 test are based on the antigens of 2019nCoV. To date, GeneMedi has produced many recombinant antigens, including Nucleocapsid (N protein), Spike protein (S protein, S1+S2 ECD), Spike protein (S1 protein), spike RBD protein, Envelope (E protein), 3C-like Proteinase. These recombinant antigens are available and ready-to-use on GeneMedi: https://www.genemedi.com/i/recombinant-2019-ncov-antigens-reagents
Reference
1.L.E.a.V.D.M. Gralinski, Return of the Coronavirus: 2019-nCoV. , Viruses, 2020. 12(2). (2020).
2.J.L. V. M. Corman, M. Witzenrath, Coronaviruses as the cause of respiratory infections, Internist (Berl) 60, 1136-1145 (2019).
3.Y. Yang, Q. Lu, M. Liu, Y. Wang, A. Zhang, N. Jalali, N. Dean, I. Longini, M.E. Halloran, B. Xu, X. Zhang, L. Wang, W. Liu, L. Fang, Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China, medRxiv, (2020).
4.J. Li, S. Li, Y. Cai, Q. Liu, X. Li, Z. Zeng, Y. Chu, F. Zhu, F. Zeng, Epidemiological and Clinical Characteristics of 17 Hospitalized Patients with 2019 Novel Coronavirus Infections Outside Wuhan, China, medRxiv, (2020).
5.C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, L. Zhang, G. Fan, J. Xu, X. Gu, Z. Cheng, T. Yu, J. Xia, Y. Wei, W. Wu, X. Xie, W. Yin, H. Li, M. Liu, Y. Xiao, H. Gao, L. Guo, J. Xie, G. Wang, R. Jiang, Z. Gao, Q. Jin, J. Wang, B. Cao, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The Lancet, 395 (2020) 497-506.
6.W.-j. Guan, Z.-y. Ni, Y. Hu, W.-h. Liang, C.-q. Ou, J.-x. He, L. Liu, H. Shan, C.-l. Lei, D.S.C. Hui, B. Du, L.-j. Li, G. Zeng, K.-Y. Yuen, R.-c. Chen, C.-l. Tang, T. Wang, P.-y. Chen, J. Xiang, S.-y. Li, J.-l. Wang, Z.-j. Liang, Y.-x. Peng, L. Wei, Y. Liu, Y.-h. Hu, P. Peng, J.-m. Wang, J.-y. Liu, Z. Chen, G. Li, Z.-j. Zheng, S.-q. Qiu, J. Luo, C.-j. Ye, S.-y. Zhu, N.-s. Zhong, Clinical characteristics of 2019 novel coronavirus infection in China, medRxiv, (2020).
7.N. Chen, M. Zhou, X. Dong, J. Qu, F. Gong, Y. Han, Y. Qiu, J. Wang, Y. Liu, Y. Wei, J.a. Xia, T. Yu, X. Zhang, L. Zhang, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, The Lancet, 395 (2020) 507-513.
8.A. Wu, Y. Peng, B. Huang, X. Ding, X. Wang, P. Niu, J. Meng, Z. Zhu, Z. Zhang, J. Wang, J. Sheng, L. Quan, Z. Xia, W. Tan, G. Cheng, T. Jiang, Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China, Cell Host Microbe, (2020).
9.R.C. A. R. Fehr, S. Perlman,, Middle East Respiratory Syndrome:Emergence of a Pathogenic Human Coronavirus, Annu Rev Med 68, 387-399 (2017).
10.X.Y. Ge, Li, J.L., Yang, X.L., Chmura, A.A., Zhu, G.,Epstein, J.H., Mazet, J.K., Hu, B., Zhang, W., Peng,C., et al. , Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor., Nature 503, 535–538 (2013).
11.M. Hoffmann, H. Kleine-Weber, N. Krüger, M. Müller, C. Drosten, S. Pöhlmann, The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells, bioRxiv, (2020).
12.C. Fan, K. Li, Y. Ding, W.L. Lu, J. Wang, ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection, medRxiv, (2020).
13.R. Channappanavar, C. Fett, M. Mack, P.P. Ten Eyck, D.K. Meyerholz, S. Perlman, Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection, The Journal of Immunology, 198 (2017) 4046-4053.
14.J. Karlberg, D.S. Chong, W.Y. Lai, Do men have a higher case fatality rate of severe acute respiratory syndrome than women do?, Am J Epidemiol, 159 (2004) 229-231.
15.Z. Li, M. Wu, J. Guo, J. Yao, X. Liao, S. Song, M. Han, J. Li, G. Duan, Y. Zhou, X. Wu, Z. Zhou, T. Wang, M. Hu, X. Chen, Y. Fu, C. Lei, H. Dong, Y. Zhou, H. Jia, X. Chen, J. Yan, Caution on Kidney Dysfunctions of 2019-nCoV Patients, medRxiv, (2020).
16.W.H. Ding YQ, Shen H, Li ZG, Geng J, Han HX, Cai JJ, Li X, Kang, W.D. W, Lu YD, Wu DH, He L, Yao KT, The clinical pathology of severe acute respiratory syndrome (SARS): a report from China., J Pathol, 2003, 200:282–289 (2003).
17.Z.L. Lang ZW, Zhang SJ, Meng X, Li JQ, Song CZ, Sun L, Zhou YS, Dwyer DE, A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). , Pathology, 2003, 35:526–531 (2003).
18.C.P. Chong PY, Ling AE, Franks TJ, Tai DY, Leo YS, Kaw GJ,, C.K. Wansaicheong G, Ean Oon LL, Teo ES, Tan KB, Nakajima, S.T. N, Travis WD, Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis., Arch Pathol Lab Med, 2004,128:195–204 (2004).
19.T.W. Chu KH, Tang CS, Lam MF, Lai FM, To KF, Fung KS, Tang HL, Yan WW, Chan HW, Lai TS, Tong KL, Lai KN, Acute renal impairment in coronavirus-associated severe acute respiratory syndrome., Kidney Int, 2005, 67:698–705 (2005).
20.H.P. Wu VC, Lin WC, Huang JW, Tsai HB, Chen YM, Wu KD, and the SARS Research Group of the National Taiwan, Acute renal failure in SARS patients: more than rhabdomyolysis. , Nephrol Dial Transplant 2004, 19:3180–3182 (2004).




