Main elements of antibody-drug conjugate (ADC): Linker (cleavable/non-cleavable, structure and mechanism)
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

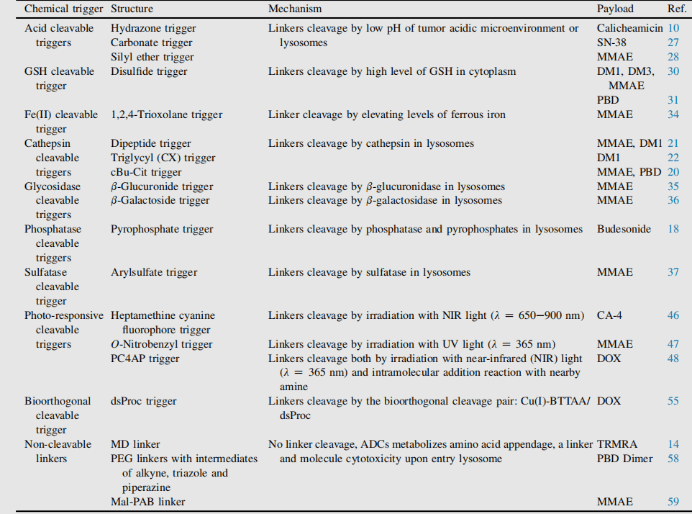
Conjugate linker is not only the molecular part forming covalent connection between antibody and small molecule payload, but also the key element with design properties in targeted drug therapy. The addition of linkers should not induce aggregation, and it is necessary to ensure acceptable PK characteristics, limit the premature release (stability) of payloads in plasma and effectively release active molecules at targeted action sites. In the process of connection, there are many conjugate companies. Connectors are divided into two types: non-cleavable linkers and cleavable linkers.1
ADC based on non-cleavable linker must be internalized, and the antibody part needs to be degraded by lysosomal protease to release active molecules. Many uncut linkers have been explored in the development of ADC. The most representative is n-succinimide-4 – (n-maleimide methyl) cyclohexane-1-carboxylate (SMCC), which is used by kadcyla.2
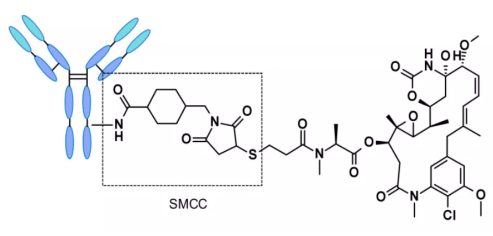
Catabolism of this structure leads to lys-smc-dm1 becoming the main tumor metabolite. In addition, drugs linked to this linker usually do not have a bystander killing/bystanerd effect, because the released catabolites have poor permeability. The current research mainly focuses on the cleavable linker.[6-7]
The use of cleavable linkers is equally feasible for the design of internalized and non internalized ADCs, because the release is triggered by the nature of the cleavage site (lysosome and / or tumor environment). Linkers can be divided into two categories: enzyme dependent and chemical (i.e. non enzyme) dependent (both have conjugate manufacturing).
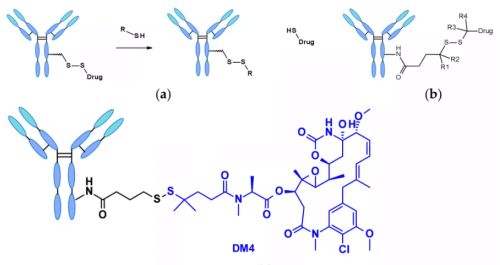
Chemically dependent linker : The linker containing disulfide bond is attacked by mercaptan to release the active load. Although the reduced form of human serum albumin (HSA) in plasma is the most abundant mercaptan, its reactivity to macromolecules is very poor.8The cytoplasm also contains high levels of glutathione (GSH), a tripeptide containing sulfhydryl, which is easy to react with s-nucleophilic protein. The difference of GSH concentration in blood (micro molar range) and cytoplasm (millimolar range) and oxidative stress caused by cancer cells contribute to the preferential release of drugs in cancer cells. Linkers containing disulfide bonds are mainly related to maytansinoid payloads. The reactivity of disulfide bonds can be adjusted by steric hindrance: α- Methyl substitution significantly affects the reduction rate and resistance to mercaptan disulfide bond exchange. For example, the linker of sar-3419 obtains the best antitumor activity of spdb-DM4 through dimethyl substitution.
Hydrazone linkers show pH dependent stability, stable at neutral pH, and hydrolyze in acidic medium (pH < 6 of endosomes and pH < 5 of lysosomes) to form corresponding ketones and hydrazine.
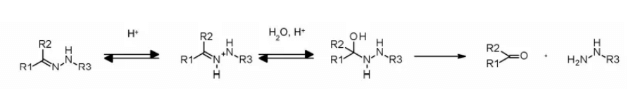
The method has been successfully applied to immu-110, which contains a cleavable acyl hydrazone linker, which is formed by the reaction of the hydrazide of 4-maleimide methylcyclohexane-1-carboxylate (MCC) with the ketone group present in adriamycin.
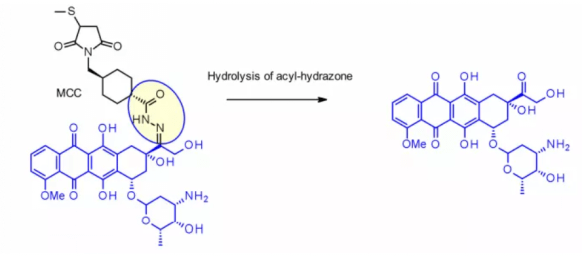
Hydrazone linkers are also often used for the payloads of the calimycin family. In this case, the release is triggered by a two-step activation process: the first step is that the acid sensitive hydrazone is hydrolyzed, and the second step is that the disulfide bond is reduced by GSH to cyclize the sulfhydryl intermediate. This linker has been used in the listed Mylotarg and besponsa, but their stability in plasma is not as stable as expected and not as attractive as other cleavable linkers.
Enzyme dependent linker: In order to limit the release of payload before internalization, so as to prevent or minimize the degradation of target cells, the protein components of lysosomes become a reasonable place to find enzymes that can degrade ADC and exist in high concentration.
Cathepsin-B
Cathepsin B is a cysteine protease, which exists in advanced endosomes and lysosomes of mammals and is overexpressed in many cancer cells. Initially, a cleavable dipeptide was used as the substrate of cathepsin B as adriamycin prodrug. This work established the dipeptide part of SAR: hydrophilic residues (citrulline or arginine) are required at P1, while lipophilic residues at P2 enhance plasma stability (phenylalanine, valine or alanine).
In addition, a self degradation spacer was introduced to promote the entry of the enzyme, thus limiting the steric hindrance of the payload: the spontaneous 1,6-elimination of p-aminobenzylcarbamate (PABA) in acidic medium, releasing carbon dioxide, p-azaquinone formamide and adriamycin. Finally, this discovery was transferred from the prodrug to the ADC field, demonstrating the antigen driven cellular activity of Val CIT and Phe Lys dipeptide linkers.4
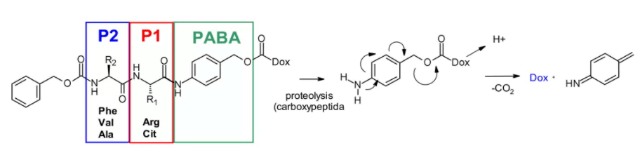
Val-Cit dipeptide is the most commonly used cleavable linker in ADCs. At present, there are up to 25 molecules in clinical stage, which may be due to its overall good plasma stability, release behavior and chemical tractability. Two approved ADC drugs (adcetris and polyvy) use the same linker MC VC PABC, which contains maleimide spacer, standard Val CIT dipeptide sequence as cathepsin substrate and PABC self degradation spacer.
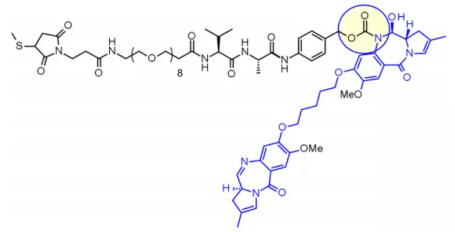
Val-Ala dipeptide is also widely used. Seven molecules are in the clinical stage. The fastest progress is loncastuximab tesirine, which includes a PEGylated spacer to balance the lipophilicity of the payload sg3199 belonging to the PBD dimer family.
Research shows that Val CIT is difficult to achieve high Dar due to precipitation and aggregation. In contrast, the Val ala linker allows Dar up to 7.4 with limited aggregation (< 10%). Compared with Val CIT, Val ala has lower hydrophobicity, which explains why this linker is excellent in lipophilic payload (such as PBD dimer). Val ala linkers of seven clinical candidate ADCs are connected to PBD.
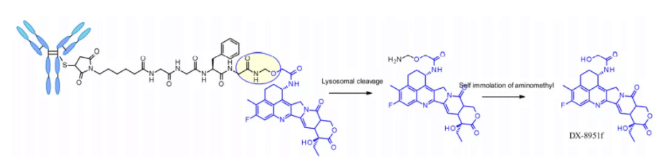
Some studies have compared the structure of Val CIT and val ala dipeptides with the payload connection of MMAE. In the case of non internalized antibodies, Val CIT and val ala linkers bound to engineered cysteine showed similar characteristics and better performance than Val Lys and val Arg analogues. In the case of anti HER2 ADC with random cysteine binding, Val ala showed less aggregation in the high Dar structure than Val CIT. On the other hand, the two linkers showed similar buffer stability, cathepsin B release efficiency, cell activity and histopathological characteristics. Tetrapeptide Gly-Gly-Phe-Gly shows all the characteristics of stable and effective cleavable linkers, which are used by the listed ADC drug enhertu. The first trimester of enhertu is a plasma stable ADC with a dar of 7.7. Protease degradation occurs in lysosomes and dx-8951f is released. It is an effective topoisomerase I inhibitor derived from exatecan. Since the linker does not contain solubilizers, reaching such a high Dar is very considerable because it contradicts the widely established principle that high Dar conjugates may have poor pharmacokinetic characteristics. The self degradation spacer used here is simple and compact hemiamination, rather than the PABC used by Val-CIT linker.
Phosphatase and pyrophosphatase
Like cathepsin, pyrophosphatase and phosphatase are hydrolases selectively expressed in lysosomes. In 2016, Merck researchers designed a linker containing phosphate and pyrophosphate to be paired with cathepsin b-sensitive Val CIT PABA to transfer glucocorticoids: phosphate / pyrophosphate partially binds between the self degrading spacer PABA and the payload. After internalization, the payload can be released in the order of cathepsin B, self degradation spacer and phosphatase (n = 1). For pyrophosphate (n = 2), another step involving pyrophosphatase may be required.
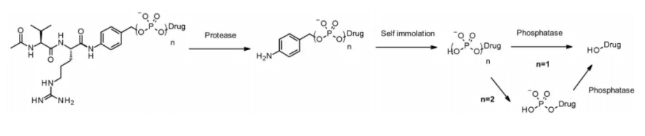
This hydrophilic and permanently charged group has the advantage of solubility. It can not only biocouple with lipophilic glucocorticoid derivatives, but also promote the purification of ADC. The residual linker in ADC is less than 0.10%. ADC containing phosphoric acid and pyrophosphoric acid has activity in vitro.
β- Glucuronidase
β- Glucuronidases are glycosidases that catalyze β- Hydrolysis of glucuronic acid residues, which are highly expressed in lysosomes and tumor stroma. Seattle genetics researchers published a groundbreaking work in 2006. Anti-CD70 ADC uses a linker containing glucuronic acid, which is attached to the self degrading spacer. This linker exhibits low levels of aggregation, high plasma stability, and strong in vivo efficacy.5
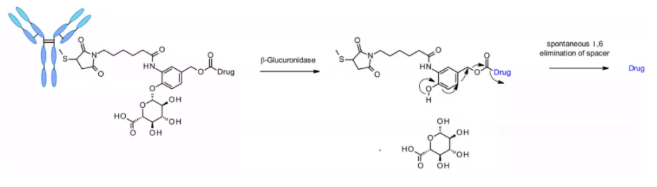
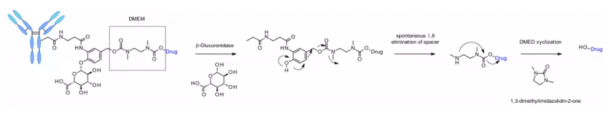
The linker is also applied to other amine containing payloads such as camptothecin analogues, sn38, ducamycin and matrine through an additional dimethylethylenediamine (DMED) self degradation spacer. Release sequence from hydrolysis β- From glucuronic acid to self degradation spacer, another cyclization reaction of DMED occurs spontaneously, forming 1,3-dimethylimidazoline-2-one, and finally releasing hydroxyl containing drugs. Due to the hydrophilicity of linker, compared with cathepsin sensitive linker, this technology makes the preparation of ADC DAR = 8 easier.
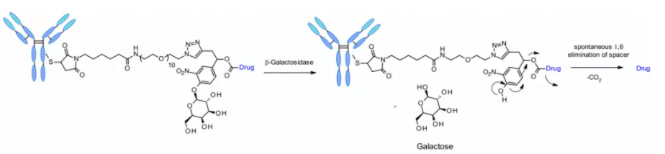
β- Galactosidase
Recently, a method of using β- Galactosidase cleaves the ADC of the linker, which contains the PEG10 spacer. The spacer was substituted by nitro to improve the self degradation rate. analogy β- Glucuronidase linker, whose dissociation mechanism involves hydrolysis β- Galactosidase moiety, which imparts hydrophilicity to chemical precursors. Another advantage is β- Galactosidase exists only in lysosomes, and β- Glucuronidase is expressed in lysosomes and in the microenvironment of solid tumors. Studies have shown that in the context of anti-HER2 ADCs releasing MMAE, it contains β- The ADC of galactosidase linker is more effective than t-dm1 in vitro and in vivo.
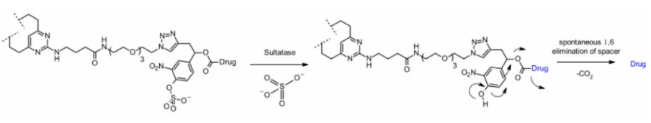
Sulfatase
Recently, there have been connectors cleaved by sulfatase, which is overexpressed in several cancer types and shows potential selectivity. The study involved anti-HER2 antibodies loaded with MMAE. Compared with the classical cleavable Val-Cit and Val-Ala linkers, the sulfatase linker showed similar efficacy on HER2+ cell lines.
Product List
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Telimomab Aritox Biosimilar, Whole Mab Adc, Anti-Cd5 Antibody: Anti-LEU1/T1 therapeutic antibody |
telimomab aritox |
NA |
CD5 |
ADC |
||
Pre-Made Telisotuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Met Antibody: Anti-AUTS9/DFNB97/HGFR/RCCP2/c-Met therapeutic antibody Drug Conjugate |
telisotuzumab vedotin |
NA |
MET |
Whole mAb ADC |
||
Pre-Made Tisotumab Vedotin Biosimilar, Whole Mab Adc, Anti-F3 Antibody: Anti-CD142/TF/TFA therapeutic antibody Drug Conjugate |
tisotumab vedotin |
NA |
F3 |
Whole mAb ADC |
||
Pre-Made Trastuzumab Deruxtecan Biosimilar, Whole Mab Adc, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2/c-ERB-2/c-ERB2 therapeutic antibody Drug Conjugate |
trastuzumab deruxtecan |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Trastuzumab Duocarmazine Biosimilar, Whole Mab Adc, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2/c-ERB-2/c-ERB2 therapeutic antibody Drug Conjugate |
trastuzumab duocarmazine |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Trastuzumab Emtansine Biosimilar, Whole Mab Adc, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2/c-ERB-2/c-ERB2 therapeutic antibody Drug Conjugate |
trastuzumab emtansine |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Tusamitamab Ravtansine Biosimilar, Whole Mab Adc, Anti-CEACAM5/CD66e Antibody: Anti-CEA therapeutic antibody Drug Conjugate |
tusamitamab ravtansine |
NA |
CEACAM5 |
Whole mAb ADC |
||
Pre-Made Upifitamab Rilsodotin Biosimilar, Whole Mab Adc, Anti-Slc34A2 Antibody: Anti-NAPI-3B/NAPI-IIb/NPTIIb/PULAM therapeutic antibody Drug Conjugate |
upifitamab rilsodotin |
NA |
SLC34A2 |
Whole mAb ADC |
||
Pre-Made Vadastuximab Talirine Biosimilar, Whole Mab Adc, Anti-Cd33 Antibody: Anti-SIGLEC-3/SIGLEC3/p67 therapeutic antibody Drug Conjugate |
vadastuximab talirine |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Vandortuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Steap1 Antibody: Anti-PRSS24/STEAP therapeutic antibody Drug Conjugate |
vandortuzumab vedotin |
NA |
STEAP1 |
Whole mAb ADC |
||
Pre-Made Vorsetuzumab Mafodotin Biosimilar, Whole Mab Adc, Anti-CD70/CD27-L Antibody: Anti-CD27LG/LPFS3/TNFSF7/TNLG8A therapeutic antibody Drug Conjugate |
vorsetuzumab mafodotin |
NA |
CD70 |
Whole mAb ADC |
||
Pre-Made Zilovertamab Vedotin Biosimilar, Whole Mab Adc, Anti-Ror1 Antibody: Anti-NTRKR1/dJ537F10.1 therapeutic antibody Drug Conjugate |
zilovertamab vedotin |
NA |
ROR1 |
Whole mAb ADC |
||
Pre-Made Zolimomab Aritox Biosimilar, Whole Mab Adc, Anti-Cd5 Antibody: Anti-LEU1/T1 therapeutic antibody |
zolimomab aritox |
NA |
CD5 |
ADC |
||
Pre-Made Actinium (225Ac) Lintuzumab Satetraxetan Biosimilar, Whole Mab Adc, Anti-Cd33 Antibody: Anti-SIGLEC-3/SIGLEC3/p67 therapeutic antibody Drug Conjugate |
actinium (225Ac) lintuzumab satetraxetan |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Anetumab Corixetan Biosimilar, Whole Mab Adc, Anti-Msln Antibody: Anti-MPF/SMRP therapeutic antibody Drug Conjugate |
anetumab corixetan |
NA |
MSLN |
Whole mAb ADC |
||
Pre-Made Anetumab Ravtansine Biosimilar, Whole Mab Adc, Anti-Msln Antibody: Anti-MPF/SMRP therapeutic antibody Drug Conjugate |
anetumab ravtansine |
NA |
MSLN |
Whole mAb ADC |
||
Pre-Made Aprutumab Ixadotin Biosimilar, Whole Mab Adc, Anti-Fgfr2 Antibody: Anti-BBDS/BEK/BFR-1/CD332/CEK3/CFD1/ECT1/JWS/K-SAM/KGFR/TK14/TK25 therapeutic antibody Drug Conjugate |
aprutumab ixadotin |
NA |
FGFR2 |
Whole mAb ADC |
||
Pre-Made Azintuxizumab Vedotin Biosimilar, Whole Mab Adc, Anti-SLAMF7/CS1 Antibody: Anti-19A/CD319/CRACC therapeutic antibody Drug Conjugate |
azintuxizumab vedotin |
NA |
SLAMF7 |
Whole mAb ADC |
||
Pre-Made Belantamab Mafodotin Biosimilar, Whole Mab Adc, Anti-Tnfrsf17 Antibody: Anti-BCM/BCMA/CD269/TNFRSF13A therapeutic antibody Drug Conjugate |
belantamab mafodotin |
NA |
TNFRSF17 |
Whole mAb ADC |
||
Pre-Made Brentuximab Vedotin Biosimilar, Whole Mab Adc, Anti-Tnfrsf8 Antibody: Anti-CD30/D1S166E/Ki-1 therapeutic antibody Drug Conjugate |
brentuximab vedotin |
NA |
TNFRSF8 |
Whole mAb ADC |
||
Pre-Made Camidanlumab Tesirine Biosimilar, Whole Mab Adc, Anti-Il2Ra Antibody: Anti-CD25/IDDM10/IL2R/IMD41/TCGFR/p55 therapeutic antibody Drug Conjugate |
camidanlumab tesirine |
NA |
IL2RA |
Whole mAb ADC |
||
Pre-Made Cantuzumab Mertansine Biosimilar, Whole Mab Adc, Anti-Muc1 Antibody: Anti-ADMCKD/ADMCKD1/ADTKD2/CA 15-3/CD227/Ca15-3/EMA/H23AG/KL-6/MAM6/MCD/MCKD/MCKD1/SEC/X/ZD/PEM/PEMT/PUM therapeutic anti |
cantuzumab mertansine |
NA |
MUC1 |
Whole mAb ADC |
||
Pre-Made Cantuzumab Ravtansine Biosimilar, Whole Mab Adc, Anti-Muc1 Antibody: Anti-ADMCKD/ADMCKD1/ADTKD2/CA 15-3/CD227/Ca15-3/EMA/H23AG/KL-6/MAM6/MCD/MCKD/MCKD1/SEC/X/ZD/PEM/PEMT/PUM therapeutic anti |
cantuzumab ravtansine |
NA |
MUC1 |
Whole mAb ADC |
||
Pre-Made Cetuximab Sarotalocan Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
cetuximab sarotalocan |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Clivatuzumab Tetraxetan Biosimilar, Whole Mab Adc, Anti-Muc1 Antibody: Anti-ADMCKD/ADMCKD1/ADTKD2/CA 15-3/CD227/Ca15-3/EMA/H23AG/KL-6/MAM6/MCD/MCKD/MCKD1/SEC/X/ZD/PEM/PEMT/PUM therapeutic an |
clivatuzumab tetraxetan |
NA |
MUC1 |
Whole mAb ADC |
||
Pre-Made Cofetuzumab Pelidotin Biosimilar, Whole Mab Adc, Anti-Ptk7 Antibody: Anti-CCK-4/CCK4 therapeutic antibody Drug Conjugate |
cofetuzumab pelidotin |
NA |
PTK7 |
Whole mAb ADC |
||
Pre-Made Coltuximab Ravtansine Biosimilar, Whole Mab Adc, Anti-Cd19 Antibody: Anti-B4/CVID3 therapeutic antibody Drug Conjugate |
coltuximab ravtansine |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Dafsolimab Setaritox Biosimilar, Whole Mab Adc, Anti-Cd3E Antibody: Anti-CD3epsilon/IMD18/T3E/TCRE therapeutic antibody Drug Conjugate |
dafsolimab setaritox |
NA |
CD3E |
Whole mAb ADC |
||
Pre-Made Datopotamab Deruxtecan Biosimilar, Whole Mab Adc, Anti-Tacstd2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody Drug Conjugate |
datopotamab deruxtecan |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Denintuzumab Mafodotin Biosimilar, Whole Mab Adc, Anti-Cd19 Antibody: Anti-B4/CVID3 therapeutic antibody Drug Conjugate |
denintuzumab mafodotin |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Depatuxizumab Mafodotin Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
depatuxizumab mafodotin |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Disitamab Vedotin Biosimilar, Whole Mab Adc, Anti-ERBB2/HER2 Antibody: Anti-CD340/neu/MLN 19/NEU/NGL/TKR1/VSCN2/c-ERB-2/c-ERB2 therapeutic antibody Drug Conjugate |
disitamab vedotin |
NA |
ERBB2 |
Whole mAb ADC |
||
Pre-Made Dorlimomab Aritox Biosimilar, Whole Mab Adc: Anti-40S Ribosomal Protein S18 therapeutic antibody |
dorlimomab aritox |
NA |
40S ribosomal protein S18 |
ADC |
||
Pre-Made Enapotamab Vedotin Biosimilar, Whole Mab Adc, Anti-Axl Antibody: Anti-ARK/JTK11/Tyro7/UFO therapeutic antibody Drug Conjugate |
enapotamab vedotin |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Enfortumab Vedotin Biosimilar, Whole Mab Adc, Anti-PVRL4/NECTIN4 Antibody: Anti-EDSS1/LNIR/PRR4/nectin-4 therapeutic antibody Drug Conjugate |
enfortumab vedotin |
NA |
PVRL4 |
Whole mAb ADC |
||
Pre-Made Farletuzumab Ecteribulin Biosimilar, Whole Mab Adc, Anti-Folr1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody Drug Conjugate |
farletuzumab ecteribulin |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Gemtuzumab Ozogamicin Biosimilar, Whole Mab Adc, Anti-Cd33 Antibody: Anti-SIGLEC-3/SIGLEC3/p67 therapeutic antibody Drug Conjugate |
gemtuzumab ozogamicin |
NA |
CD33 |
Whole mAb ADC |
||
Pre-Made Glembatumumab Vedotin Biosimilar, Whole Mab Adc, Anti-Gpnmb Antibody: Anti-HGFIN/NMB/PLCA3 therapeutic antibody Drug Conjugate |
glembatumumab vedotin |
NA |
GPNMB |
Whole mAb ADC |
||
Pre-Made Grisnilimab Setaritox Biosimilar, Whole Mab Adc, Anti-Cd7 Antibody: Anti-GP40/LEU-9/TP41/Tp40 therapeutic antibody Drug Conjugate |
grisnilimab setaritox |
NA |
CD7 |
Whole mAb ADC |
||
Pre-Made Iladatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody Drug Conjugate |
iladatuzumab vedotin |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Indatuximab Ravtansine Biosimilar, Whole Mab Adc, Anti-Sdc1 Antibody: Anti-CD138/SDC/SYND1/syndecan therapeutic antibody Drug Conjugate |
indatuximab ravtansine |
NA |
SDC1 |
Whole mAb ADC |
||
Pre-Made Indusatumab Vedotin Biosimilar, Whole Mab Adc, Anti-Gucy2C Antibody: Anti-DIAR6/GC-C/GUC2C/MECILIL/STAR therapeutic antibody Drug Conjugate |
indusatumab vedotin |
NA |
GUCY2C |
Whole mAb ADC |
||
Pre-Made Inotuzumab Ozogamicin Biosimilar, Whole Mab Adc, Anti-Cd22 Antibody: Anti-SIGLEC-2/SIGLEC2 therapeutic antibody Drug Conjugate |
inotuzumab ozogamicin |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Labetuzumab Govitecan Biosimilar, Whole Mab Adc, Anti-CEACAM5/CD66e Antibody: Anti-CEA therapeutic antibody Drug Conjugate |
labetuzumab govitecan |
NA |
CEACAM5 |
Whole mAb ADC |
||
Pre-Made Ladiratuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Slc39A6 Antibody: Anti-LIV-1/LIV1/ZIP6 therapeutic antibody Drug Conjugate |
ladiratuzumab vedotin |
NA |
SLC39A6 |
Whole mAb ADC |
||
Pre-Made Laprituximab Emtansine Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
laprituximab emtansine |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lifastuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Slc34A2 Antibody: Anti-NAPI-3B/NAPI-IIb/NPTIIb/PULAM therapeutic antibody Drug Conjugate |
lifastuzumab vedotin |
NA |
SLC34A2 |
Whole mAb ADC |
||
Pre-Made Loncastuximab Tesirine Biosimilar, Whole Mab Adc, Anti-Cd19 Antibody: Anti-B4/CVID3 therapeutic antibody Drug Conjugate |
loncastuximab tesirine |
NA |
CD19 |
Whole mAb ADC |
||
Pre-Made Lonigutamab Ugodotin Biosimilar, Whole Mab Adc, Anti-Igf1R Antibody: Anti-CD221/IGFIR/IGFR/JTK13 therapeutic antibody Drug Conjugate |
lonigutamab ugodotin |
NA |
IGF1R |
Whole mAb ADC |
||
Pre-Made Lorvotuzumab Mertansine Biosimilar, Whole Mab Adc, Anti-Ncam1 Antibody: Anti-CD56/MSK39/NCAM therapeutic antibody Drug Conjugate |
lorvotuzumab mertansine |
NA |
NCAM1 |
Whole mAb ADC |
Reference
1. Su Z , Xiao D , Xie F , et al. Antibodydrug conjugates: Recent advances in linker chemistry[J]. Acta Pharmaceutica Sinica B, 2021.
2. Chen Y , Kim M T , Zheng L , et al. Structural Characterization of Cross-Linked Species in Trastuzumab Emtansine (Kadcyla)[J]. Bioconjugate Chemistry, 2016.
3. Seki H , Walsh S J , Bargh J D , et al. Rapid and robust cysteine bioconjugation with vinylheteroarenes. 2021.
4. Kostova V, Désos P, Starck JB, Kotschy A. The Chemistry Behind ADCs. Pharmaceuticals (Basel). 2021;14(5):442.
5. Kostova, V.; Désos, P.; Starck, J.-B.; Kotschy, A. The Chemistry Behind ADCs. Pharmaceuticals 2021, 14, 442.
6. Singh A P , Sharma S , Shah D K . Quantitative characterization of in vitro bystander effect of antibody-drug conjugates[J]. J Pharmacokinet Pharmacodyn, 2016, 43(6):567-582.
7. Aleksandr V. Yurkovetskiy;Natalya D. Bodyak;Mao Yin;A Novel Antibody–Drug Conjugate Platform Featuring High Drug Loading and a Controlled Bystander Effect
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




