Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

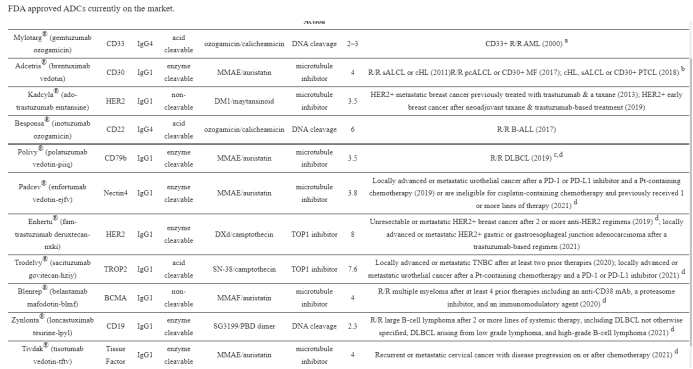
1. Mylotarg® (gemtuzumab ozogamicin) from Wyeth/Pfizer was the first ADC to reach the market. It is composed of a recombinant humanized anti-CD33 mAb (IgG4κ antibody hP67.6) covalently attached to a calicheamicin derived payload (N-acetyl-γ-calicheamicin 1,2-dimethyl hydrazine dichloride) via a pH-sensitive hydrazone linker.
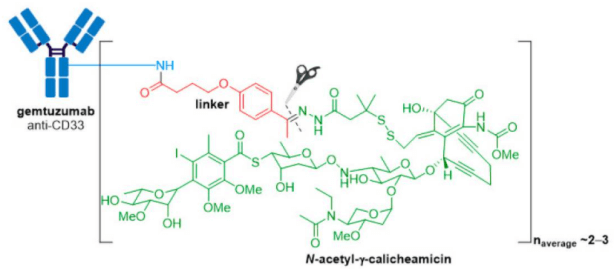
2. Adcetris® (brentuximab vedotin) from Seagen (formerly Seattle Genetics), containing a CD30-specific mAb conjugated to monomethyl auristatin E (MMAE), received FDA approval in 2011, making it the second ADC to enter the oncology market.
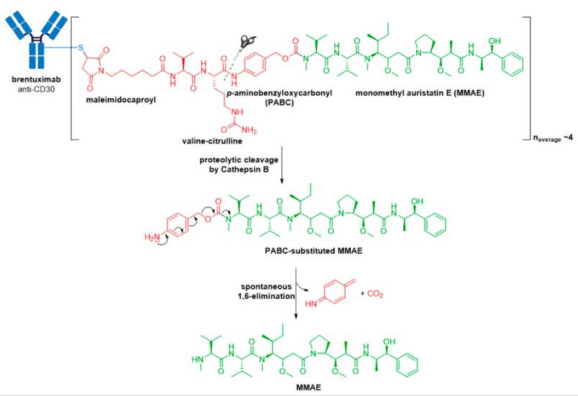
3. In 2013, Kadcyla® (ado-trastuzumab emtansine), developed and marketed by Genentech/Roche, revolutionized the field of ADCs by becoming the first ADC approved for the treatment of solid tumors. It is indicated as an adjuvant (after surgery) treatment for HER2+ early breast cancer in patients who previously received trastuzumab (Herceptin®) and a taxane, separately or in combination
4. Besponsa® (inotuzmab ozogamicin (Pfizer/Wyeth)) obtained FDA approval in 2017 and is directed against CD22+ B-cell acute lymphoblastic leukemia (B-ALL). The first difference lies in the mAb and thus the antigen target and cancer indication. The recombinant humanized monoclonal IgG4 antibody (G544) employed in Besponsa® is selective for CD22 expressed on B cells in all patients with mature B-ALL, and >90% of patients with precursor B-ALL. n.
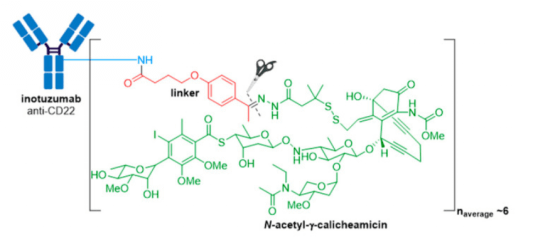
5. Polivy and Padcev Polivy® is an anti-CD79b ADC developed by Genentech/Roche using a proprietary technology developed by Seagen. It is indicated in combination with bendamustine and rituximab for treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), an aggressive type of non-Hodgkin lymphoma, who have received at least two prior therapies. This indication was granted accelerated approval based on a complete response rate. Polivy® has an approximate DAR of 3.5 molecules of MMAE attached to each antibody.
Padcev®, produced and marketed by Astellas Pharma Inc. and Seagen is a Nectin4-directed ADC. It was first granted accelerated approval in 2019 for treatment of adults with locally advanced or metastatic urothelial cancer who have previously received a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor, and a platinum-containing therapy. In 2021, this indication was granted regular approval and Padcev® was granted accelerated approval for patients which are ineligible for cisplatin-containing chemotherapy and have previously received one or more prior lines of therapy. Padcev® is comprised of a fully humanized anti-Nectin4 IgG1κ mAb (AGS-22C3) produced by mammalian (Chinese hamster ovary) cells, and has an approximate DAR of 3.8.
6 .Enhertu® (fam-trastuzumab deruxtecan-nxki), developed by Daichi Sankyo/AstraZeneca, was granted accelerated FDA approval in December 2019 for treatment of adult patients with unresectable or metastatic HER2+ breast cancer who have received two or more prior anti-HER2 based regimens. The ADC is comprised of an anti-HER2 antibody, a protease cleavable tetrapeptide-based linker, and DXd as the drug payload.
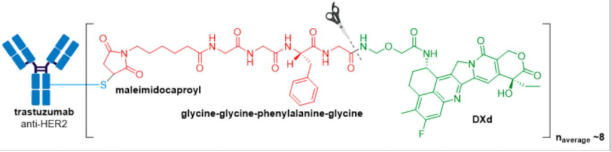
In April 2020, Trodelvy® received accelerated FDA approval for treatment of patients with locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for metastatic disease. Trodelvy® consists of a fully humanized hRS7 IgG1κ antibody targeted against TROP2 (trophoblast antigen 2) conjugated to SN-38, the active metabolite of irinotecan via an acid-sensitive hydrolysable linker called CL2A.
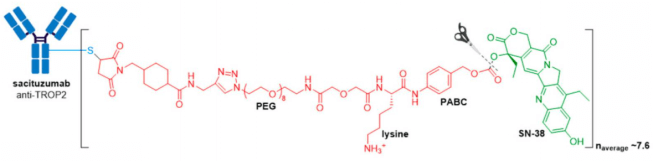
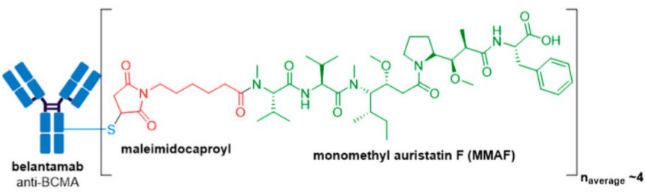
GlaxoSmithKline’s ADC, Blenrep® (belantamab mafodotin-blmf), is the first approved anti-BCMA (B-cell maturation antigen) therapy. It was granted accelerated FDA approval in August 2020 for treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies, including an anti-CD38 mAb, a proteasome inhibitor, and an immunomodulatory agent. Blenrep® consists of an afucosylated humanized IgG1 mAb conjugated to the tubulin inhibitor, monomethyl auristatin F (MMAF) via a non-cleavable maleimidocaproyl linker. In addition to MMAF-induced apoptosis, secondary antitumor activity results from tumor cell lysis through ADCC and ADCP effector functions.
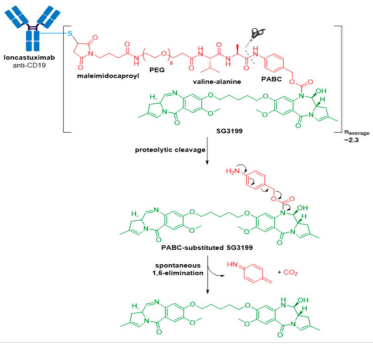
Zynlonta® (loncastuximab tesirine-lpyl) developed by ADC Therapeutics is a CD19-directed ADC indicated for treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL), not otherwise specified DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma. It was granted accelerated approval for medical use by the FDA in April 2021. Zynlonta® is composed of a humanized IgG1κ mAb conjugated to SG3199, a cytotoxic pyrrolobenzodiazepine (PBD) dimer alkylating agent, through a protease-cleavable valine-alanine linker.
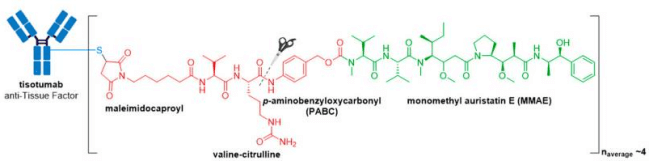
In late September 2021, the FDA granted accelerated approval to Tivdak® (tisotumab vedotin-tftv), deeming it the most recently approved ADC on the market. Tivdak®, co-developed by Seagen and Genmab, is the first and only approved ADC indicated for treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy . Tivdak® is a Tissue Factor (TF) directed ADC comprised of a human anti-TF IgG1κ antibody conjugated to MMAE via the same protease-cleavable mc-vc-PABC linker construct employed in Adcetris®, Polivy®, and Padcev®. As for these previously discussed ADCs, Tivdak® carries an average of four MMAE molecules per mAb. Furthermore, in vitro studies have demonstrated that this ADC also mediates ADCP and ADCC effector functions, thus providing multimodal antitumor activity.
As ADCs have undergone clinical development, it has become clear that the rules applying to standard chemotherapy or antibody-based therapies on their own do not necessarily apply to ADCs. ADCs are modular in nature, with interchangeable components that can be altered in a strategic fashion to improve both their efficacy and toxicity profiles. 1
Reference:
1. Mol Cancer Ther (2021) 20 (5): 885–895.
Product List
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Losatuxizumab Vedotin Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
losatuxizumab vedotin |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lupartumab Amadotin Biosimilar, Whole Mab Adc, Anti-Lypd3 Antibody: Anti-C4.4A therapeutic antibody Drug Conjugate |
lupartumab amadotin |
NA |
LYPD3 |
Whole mAb ADC |
||
Pre-Made Mipasetamab Uzoptirine Biosimilar, Whole Mab Adc, Anti-Axl Antibody: Anti-ARK/JTK11/Tyro7/UFO therapeutic antibody Drug Conjugate |
mipasetamab uzoptirine |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Mirvetuximab Soravtansine Biosimilar, Whole Mab Adc, Anti-Folr1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody Drug Conjugate |
mirvetuximab soravtansine |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Mirzotamab Clezutoclax Biosimilar, Whole Mab Adc, Anti-Cd276 Antibody: Anti-4Ig-B7-H3/B7-H3/B7H3/B7RP-2 therapeutic antibody Drug Conjugate |
mirzotamab clezutoclax |
NA |
CD276 |
Whole mAb ADC |
||
Pre-Made Naratuximab Emtansine Biosimilar, Whole Mab Adc, Anti-Cd37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody Drug Conjugate |
naratuximab emtansine |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Patritumab Deruxtecan Biosimilar, Whole Mab Adc, Anti-ERBB3/Erbb-3 Antibody: Anti-ErbB-3/FERLK/HER3/LCCS2/MDA-BF-1/VSCN1/c-erbB-3/c-erbB3/erbB3-S/p180-ErbB3/p45-sErbB3/p85-sErbB3 therapeutic |
patritumab deruxtecan |
NA |
ERBB3 |
Whole mAb ADC |
||
Pre-Made Pelgifatamab Corixetan Biosimilar, Whole Mab Adc, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody Drug Conjugate |
pelgifatamab corixetan |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Pinatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd22 Antibody: Anti-SIGLEC-2/SIGLEC2 therapeutic antibody Drug Conjugate |
pinatuzumab vedotin |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Pivekimab Sunirine Biosimilar, Whole Mab Adc, Anti-Il3Ra Antibody: Anti-CD123/IL3R/IL3RX/IL3RY/hIL-3Ra therapeutic antibody Drug Conjugate |
pivekimab sunirine |
NA |
IL3RA |
Whole mAb ADC |
||
Pre-Made Polatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody Drug Conjugate |
polatuzumab vedotin |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Praluzatamab Ravtansine Biosimilar, Whole Mab Adc, Anti-Alcam Antibody: Anti-CD166/MEMD therapeutic antibody Drug Conjugate |
praluzatamab ravtansine |
NA |
ALCAM |
Whole mAb ADC |
||
Pre-Made Rolinsatamab Talirine Biosimilar, Whole Mab Adc, Anti-Prlr Antibody: Anti-HPRL/MFAB/RI-PRLR/hPRLrI therapeutic antibody Drug Conjugate |
rolinsatamab talirine |
NA |
PRLR |
Whole mAb ADC |
||
Pre-Made Rosopatamab Tetraxetan Biosimilar, Whole Mab Adc, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody Drug Conjugate |
rosopatamab tetraxetan |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Rovalpituzumab Tesirine Biosimilar, Whole Mab Adc, Anti-Dll3 Antibody: Anti-SCDO1 therapeutic antibody Drug Conjugate |
rovalpituzumab tesirine |
NA |
DLL3 |
Whole mAb ADC |
||
Pre-Made Sacituzumab Govitecan Biosimilar, Whole Mab Adc, Anti-Tacstd2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody Drug Conjugate |
sacituzumab govitecan |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Samrotamab Vedotin Biosimilar, Whole Mab Adc, Anti-Lrrc15 Antibody: Anti-LIB therapeutic antibody Drug Conjugate |
samrotamab vedotin |
NA |
LRRC15 |
Whole mAb ADC |
||
Pre-Made Serclutamab Talirine Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
serclutamab talirine |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Sirtratumab Vedotin Biosimilar, Whole Mab Adc, Anti-Slitrk6 Antibody: Anti-DFNMYP therapeutic antibody Drug Conjugate |
sirtratumab vedotin |
NA |
SLITRK6 |
Whole mAb ADC |
||
Pre-Made Sofituzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Muc16 Antibody: Anti-CA125 therapeutic antibody Drug Conjugate |
sofituzumab vedotin |
NA |
MUC16 |
Whole mAb ADC |
Reference
1. Mol Cancer Ther (2021) 20 (5): 885–895.
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




