Review for ADC production, quality control and functional assay
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

SDS-PAGE
We need run SDS-PAGE(reducing and non-reducing DTT) to see the integrity of antibody and Preliminary observation of the connection of small molecules. In general, we can see that the conjugated antibody will shift upward compared with the naked antibody
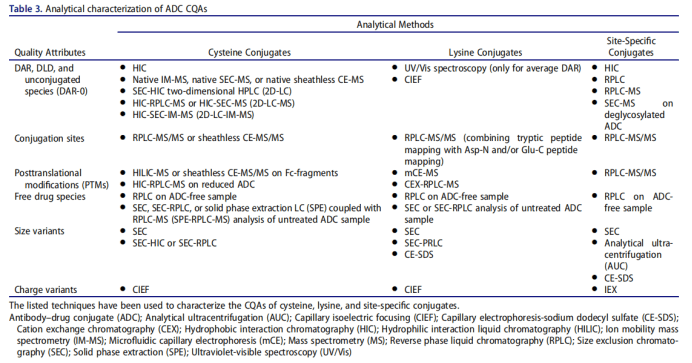
DAR (methods and standard)
ADC drugs are essentially a mixture, which is composed of mAbs connecting different numbers of small molecule drugs. Dar represents the average number of small molecule drugs connected to each mAb. Dar directly affects the efficacy and safety of ADC drugs. In the drug development stage, the variation range of DAR value should be minimized. The coupling sites of ADC drugs are divided into amino groups on lysine residues and sulfhydryl groups on cysteine residues. The DAR coupled by lysine is often small, but there are many potential coupling sites. The coupling reaction is random and the product heterogeneity is large; There are 4 pairs of inter chain disulfide bonds used in the development of ADC drugs. The antibody converts the inter chain disulfide bond into free cysteine residues through partial reduction. The sulfhydryl group in the cysteine residues reacts with the maleimide group in the linker to form ADC. Currently, most ADCs in use or under development rely on interchain disulfide cysteines for conjugation, in which the 4 (IgG1 and IgG4) or 6 (IgG2) interchain disulfide bonds are reduced by an excess reducing agent, namely tris(2-carboxyethyl)phosphine or dithiothreitol.153 This spares disruption of intrachain disulfide bonds while freeing sulfhydryl groups from cysteine residues participating in interchain disulfide bonds (Figure 3e). The resulting product is a mixture of ADCs containing 0–8 drugs per parent IgG1 or IgG4 and 0– 12 per IgG2, with predominantly even numbered DAR (0, 2, 4, 6, 8, 10, 12) species within the ADC mixture.
Ultraviolet / visible spectroscopy (UV / VIS)
UV / Vis spectroscopy is the simplest and stable method to detect Dar value. This method requires antibodies and small molecule drugs to have different maximum absorption wavelengths, and calculate their concentrations respectively to obtain the DAR value of ADC, which is suitable for a variety of ADCs.
Chromatography
Chromatography includes hydrophobic interaction chromatography (HIC) and reverse phase high performance liquid chromatography (RP-HPLC), which are suitable for the determination of cysteine coupled ADC. Hydrophobic interaction chromatography can separate the components with different Dar values according to the difference of hydrophobicity, and maintain the structural integrity of ADC molecules; Reversed phase high performance liquid chromatography needs to reduce the antibody to obtain light and heavy chains before analysis. It can be used to supplement and verify the results of hydrophobic interaction chromatography, and is suitable for mass spectrometry.
Mass spectrometry
Mass spectrometry is suitable for the determination of DAR value of lysine coupled ADC, including liquid chromatography tandem mass spectrometry and MALDI-TOF-MS. Lysine coupled ADC has strong heterogeneity, which increases the difficulty of mass spectrum analysis. Usually, additional pretreatment of ADC is required before determination, such as deglycosylation and removal of C-terminal lysine heterogeneity.
Cytotoxity assay of ADC
After the DAR of antibody is determined, the cytotoxicity experiment of ADC needs to be carried out. The cells are treated with different doses of ADC and their EC50 is analyzed. In the cytotoxicity experiment in vitro, the cells in the control group knocked out the target gene, so the antibody lost the role of localization and could not kill tumor cells, so it did not have the regularity of dose. However, from the treatment results of cells that have never been knocked out, they generally have better killing and EC50.
Cat No. | Products Name (INN Index) | INN Name | Previous Name | Target | Format | Order |
|---|---|---|---|---|---|---|
Pre-Made Losatuxizumab Vedotin Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
losatuxizumab vedotin |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Lupartumab Amadotin Biosimilar, Whole Mab Adc, Anti-Lypd3 Antibody: Anti-C4.4A therapeutic antibody Drug Conjugate |
lupartumab amadotin |
NA |
LYPD3 |
Whole mAb ADC |
||
Pre-Made Mipasetamab Uzoptirine Biosimilar, Whole Mab Adc, Anti-Axl Antibody: Anti-ARK/JTK11/Tyro7/UFO therapeutic antibody Drug Conjugate |
mipasetamab uzoptirine |
NA |
AXL |
Whole mAb ADC |
||
Pre-Made Mirvetuximab Soravtansine Biosimilar, Whole Mab Adc, Anti-Folr1 Antibody: Anti-FBP/FOLR/FRalpha/NCFTD therapeutic antibody Drug Conjugate |
mirvetuximab soravtansine |
NA |
FOLR1 |
Whole mAb ADC |
||
Pre-Made Mirzotamab Clezutoclax Biosimilar, Whole Mab Adc, Anti-Cd276 Antibody: Anti-4Ig-B7-H3/B7-H3/B7H3/B7RP-2 therapeutic antibody Drug Conjugate |
mirzotamab clezutoclax |
NA |
CD276 |
Whole mAb ADC |
||
Pre-Made Naratuximab Emtansine Biosimilar, Whole Mab Adc, Anti-Cd37 Antibody: Anti-GP52-40/TSPAN26 therapeutic antibody Drug Conjugate |
naratuximab emtansine |
NA |
CD37 |
Whole mAb ADC |
||
Pre-Made Patritumab Deruxtecan Biosimilar, Whole Mab Adc, Anti-ERBB3/Erbb-3 Antibody: Anti-ErbB-3/FERLK/HER3/LCCS2/MDA-BF-1/VSCN1/c-erbB-3/c-erbB3/erbB3-S/p180-ErbB3/p45-sErbB3/p85-sErbB3 therapeutic |
patritumab deruxtecan |
NA |
ERBB3 |
Whole mAb ADC |
||
Pre-Made Pelgifatamab Corixetan Biosimilar, Whole Mab Adc, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody Drug Conjugate |
pelgifatamab corixetan |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Pinatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd22 Antibody: Anti-SIGLEC-2/SIGLEC2 therapeutic antibody Drug Conjugate |
pinatuzumab vedotin |
NA |
CD22 |
Whole mAb ADC |
||
Pre-Made Pivekimab Sunirine Biosimilar, Whole Mab Adc, Anti-Il3Ra Antibody: Anti-CD123/IL3R/IL3RX/IL3RY/hIL-3Ra therapeutic antibody Drug Conjugate |
pivekimab sunirine |
NA |
IL3RA |
Whole mAb ADC |
||
Pre-Made Polatuzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Cd79B Antibody: Anti-AGM6/B29/IGB therapeutic antibody Drug Conjugate |
polatuzumab vedotin |
NA |
CD79B |
Whole mAb ADC |
||
Pre-Made Praluzatamab Ravtansine Biosimilar, Whole Mab Adc, Anti-Alcam Antibody: Anti-CD166/MEMD therapeutic antibody Drug Conjugate |
praluzatamab ravtansine |
NA |
ALCAM |
Whole mAb ADC |
||
Pre-Made Rolinsatamab Talirine Biosimilar, Whole Mab Adc, Anti-Prlr Antibody: Anti-HPRL/MFAB/RI-PRLR/hPRLrI therapeutic antibody Drug Conjugate |
rolinsatamab talirine |
NA |
PRLR |
Whole mAb ADC |
||
Pre-Made Rosopatamab Tetraxetan Biosimilar, Whole Mab Adc, Anti-FOLH1/GCPII Antibody: Anti-FGCP/FOLH/GCP2/NAALAD1/PSM/PSMA/mGCP therapeutic antibody Drug Conjugate |
rosopatamab tetraxetan |
NA |
FOLH1 |
Whole mAb ADC |
||
Pre-Made Rovalpituzumab Tesirine Biosimilar, Whole Mab Adc, Anti-Dll3 Antibody: Anti-SCDO1 therapeutic antibody Drug Conjugate |
rovalpituzumab tesirine |
NA |
DLL3 |
Whole mAb ADC |
||
Pre-Made Sacituzumab Govitecan Biosimilar, Whole Mab Adc, Anti-Tacstd2 Antibody: Anti-EGP-1/EGP1/GA733-1/GA7331/GP50/M1S1/TROP2 therapeutic antibody Drug Conjugate |
sacituzumab govitecan |
NA |
TACSTD2 |
Whole mAb ADC |
||
Pre-Made Samrotamab Vedotin Biosimilar, Whole Mab Adc, Anti-Lrrc15 Antibody: Anti-LIB therapeutic antibody Drug Conjugate |
samrotamab vedotin |
NA |
LRRC15 |
Whole mAb ADC |
||
Pre-Made Serclutamab Talirine Biosimilar, Whole Mab Adc, Anti-Egfr Antibody: Anti-ERBB/ERBB1/ERRP/HER1/NISBD2/PIG61/mENA therapeutic antibody Drug Conjugate |
serclutamab talirine |
NA |
EGFR |
Whole mAb ADC |
||
Pre-Made Sirtratumab Vedotin Biosimilar, Whole Mab Adc, Anti-Slitrk6 Antibody: Anti-DFNMYP therapeutic antibody Drug Conjugate |
sirtratumab vedotin |
NA |
SLITRK6 |
Whole mAb ADC |
||
Pre-Made Sofituzumab Vedotin Biosimilar, Whole Mab Adc, Anti-Muc16 Antibody: Anti-CA125 therapeutic antibody Drug Conjugate |
sofituzumab vedotin |
NA |
MUC16 |
Whole mAb ADC |
View the Knowledge base of Antibody-drug Conjugate (ADC):
– What is antibody-drug conjugate (ADC)?
– Antibody-drug conjugate (ADC) in clinical application (Approved/BLA, phaseI/II/III)
– Main elements of antibody-drug conjugate (ADC): Antibodies and their targets
– Main elements of antibody-drug conjugate (ADC):Linker (cleavable/non-cleavable, structure and mechanism)
– Main elements of antibody-drug conjugate (ADC):Toxins/Payloads (Classification and function)
– Toxins/Payloads (Classification and function) of Microtubule destroying drug
– Toxins/Payloads (Classification and function) of DNA damage drugs
– Toxins/Payloads (Classification and function) of Innovative drugs
– Biological coupling technology Chemical based specific in situ antibody modification
– Endogenous coupling of amino acids and Disulfide re bridging strategy
– Glycan coupling
– Site specific biological coupling of engineered antibodies and Enzymatic method
– Biological coupling with engineered unnatural amino acids
– Review for ADC production, quality control and functional assay
– Product data of ADC




