Format of bispecific antibodies (BsAbs)-scFv-Fab IgG
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

The XmAb enables alterations with desirable effects to the Fc domain of the antibodies. The modification increases affinity to the neonatal Fc receptor which prevents the antibody from degradation. Hence, this interaction extends the antibody’s halflife of this therapeutic drug. In order to construct the XmAb format an antibody heavy and light chain and a scFv Fcfusion were subcloned into vectors. The scFv and Fc region were connected with a GS-linker. The Fc region was altered with substitutions in order to increase the differences in pI between the two heavy chains. This would increase the pI differences between homodimers and heterodimers, which would then facilitate the purification of heterodimers. For the production of the proteins, plasmids encoding all chains were co-transfected into HEK cells. The antibody was further purified using protein A chromatography and ion exchange chromatography. Due to alterations in the Fc domain, pI differences can be used in the purification. The risk of immunogenicity was also minimized by the XmAb.
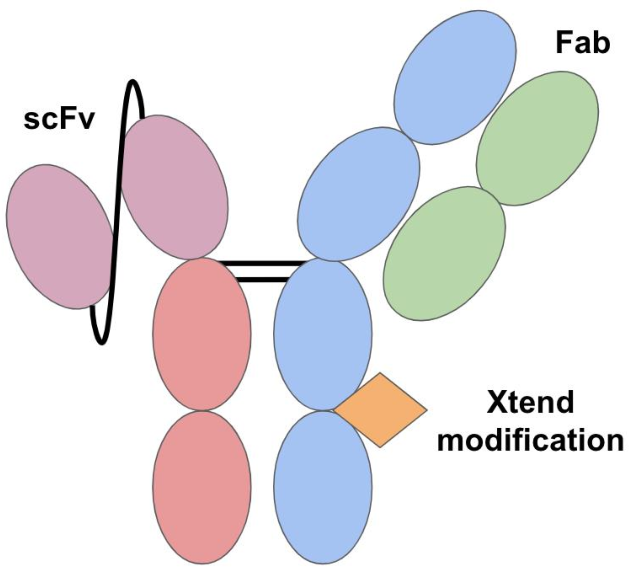
Formats of bispecific antibodies (BsAbs)
Many formats have been developed for BsAb generation as listed in the following table.
| Format | Schematic structure | Description | Example BsAb | Trademark | Company |
|---|---|---|---|---|---|
| tandem VHH | Tandem VHH fragment-based BsAb | N/A | |||
| tandem scFv |  | Tandem ScFv fragment-based BsAb | AMG330 | BiTETM | Amgen |
| Dual-affinity re-targeting antibody |  | Tandem domain-exchanged Fv (can also be used to fuse with Fc domain to create whole Abs) | Flotetuzumab | DARTTM | Macrogenics |
| Diabody |  | dimer of single-chain Fv (scFv) fragment | vixtimotamab | ReSTORETM | Amphivena Therapeutics |
| (scFv)2-Fab |  | a Fab domain and two scFv domains bind | A-337 | ITabTM | Generon/EVIVE Biotech |
| Rat–mouse hybrid IgG |  | Full-size IgG-like half antibodies from two different species | Catumaxomab | TriomabTM | Trion Pharma |
| Hetero heavy chain, Common light chain |  | Hetero heavy chain, Common light chain | Emicizumab | ART-IgTM | Genentech/ Chugai/Roche |
| Controlled Fab arm exchange | 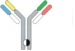 | Recombin the parental half antibodies | JNJ-64007957 | DuobodyTM | Genmab/ Janssen |
| Hetero H, forced HL IgG1 | 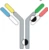 | KIH technology for heterodimerization of 2 distinct H chains, replacing the native disulfide bond in one of the CH1-CL interfaces with an engineered disulfide bond to enhance the cognate of H and L paring | MEDI5752 | DuetMabTM | MedImmune/ AstraZeneca |
| cH IgG1 | 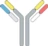 | Identical heavy chains; 2 different light chains: one kappa (κ) and one lambda (λ) | NI-1701 | κλ bodyTM | Novimmune SA |
| Hetero H, CrossMab | 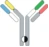 | KIH technology; domain crossover of immunoglobulin domains in the Fab region | Vanucizumab | CrossMabTM | Roche |
| scFv-Fab IgG | 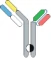 | Fab-Fc; ScFv-Fc | Vibecotamab; M802 | XmabTM (the engineered Fc to enhance the generation of heterodimeric Fc); YBODYTM | Xencor/Amgen; YZYBio |
| VH1-VH2-CH1-Fc1(G1) x VL2-VL1-CL-Fc2(G1) | 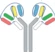 | 2 binding motif in one half antibody | SAR440234 | CODV-IgTM | Sanofi |
| VL1-CL1-VH2-CH2-Fc x VH1-CH1 x VL2-CL2 | 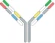 | 2 binding motif in one half antibody | EMB-01 | FIT-IgTM | EPIMAB BIOTHERAPEUTICS |
| VH-1-TCR Cα x VL-1-TCR Cβ; VH-2-CH-2-Fc x VL-2-CL-2 |  | KIH technology; TCR Cα/Cβ is used to substitute the CH1 and CL domain in one arm | WuXibodyTM | WuXi Biologics | |
| C-terminal linker of Fc |  | Link the other molecules at the C-terminal of Fc | APVO442 | ADAPTIR-FLEXTM | Aptevo Therapeutics |
| Fc antigen binding site |  | 2 natural binding sites; 2 additional binding sites in the Fc loop | FS118 | mAb2 | F-star Therapeutics |




