Format of bispecific antibodies (BsAbs)-Tandem VHH
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
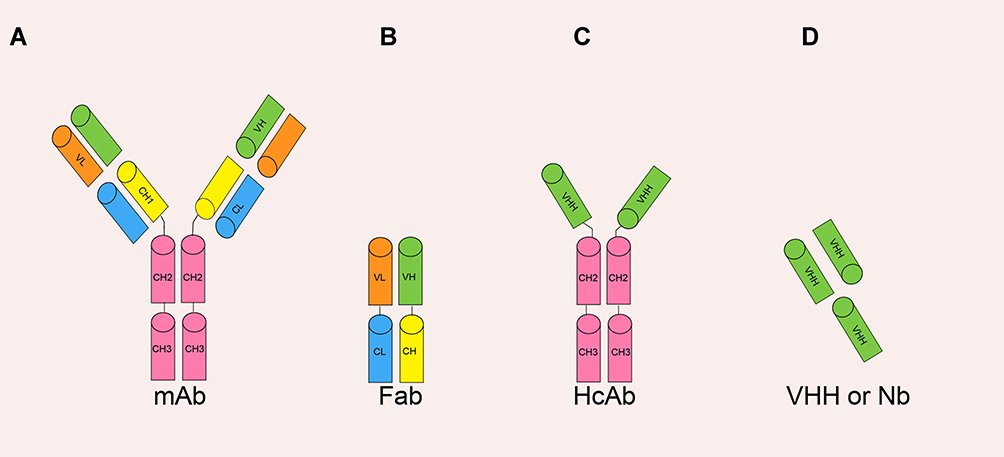
Single domain antibody (Nanobody)
SOCAIL MEDIA

Since 1975, monoclonal antibodies have been successfully used for the diagnosis and therapeutics. In 1993, heavy-chain-only antibodies which bind to antigens was discovered in camelids. The variable domain of heavy chain antibody (VHH) was called as nanobodies and it was anticipated as the better therapeutic antibodies in future due to its high stability, refoldability, and manipulable characteristics. VHH has also been effectively used for the diagnosis, bioimaging, immunoassay and affinity purification. The production of nanobodies is simple and cost effective compared to the polyclonal antibodies. Besides, Nbs are stable in a wide range of temperatures and pH levels. Nanobodies are also compatible with genetic engineering methods, which allow scaffolding and alteration of amino acids to improve binding. Relating to structure, the hydrophilic side of nanobodies, that is not present in conventional antibodies, means they do not have issues with solubility and aggregation. The conventional antibodies do not bind well in grooves or cavities on the surface of the antigen. But, Nanobodies bind with the clefts of the active site of antigen.
Formats of bispecific antibodies (BsAbs)
Many formats have been developed for BsAb generation as listed in the following table.
| Format | Schematic structure | Description | Example BsAb | Trademark | Company |
|---|---|---|---|---|---|
| tandem VHH | Tandem VHH fragment-based BsAb | N/A | |||
| tandem scFv |  | Tandem ScFv fragment-based BsAb | AMG330 | BiTETM | Amgen |
| Dual-affinity re-targeting antibody |  | Tandem domain-exchanged Fv (can also be used to fuse with Fc domain to create whole Abs) | Flotetuzumab | DARTTM | Macrogenics |
| Diabody |  | dimer of single-chain Fv (scFv) fragment | vixtimotamab | ReSTORETM | Amphivena Therapeutics |
| (scFv)2-Fab |  | a Fab domain and two scFv domains bind | A-337 | ITabTM | Generon/EVIVE Biotech |
| Rat–mouse hybrid IgG |  | Full-size IgG-like half antibodies from two different species | Catumaxomab | TriomabTM | Trion Pharma |
| Hetero heavy chain, Common light chain |  | Hetero heavy chain, Common light chain | Emicizumab | ART-IgTM | Genentech/ Chugai/Roche |
| Controlled Fab arm exchange | 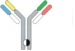 | Recombin the parental half antibodies | JNJ-64007957 | DuobodyTM | Genmab/ Janssen |
| Hetero H, forced HL IgG1 | 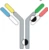 | KIH technology for heterodimerization of 2 distinct H chains, replacing the native disulfide bond in one of the CH1-CL interfaces with an engineered disulfide bond to enhance the cognate of H and L paring | MEDI5752 | DuetMabTM | MedImmune/ AstraZeneca |
| cH IgG1 | 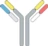 | Identical heavy chains; 2 different light chains: one kappa (κ) and one lambda (λ) | NI-1701 | κλ bodyTM | Novimmune SA |
| Hetero H, CrossMab | 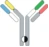 | KIH technology; domain crossover of immunoglobulin domains in the Fab region | Vanucizumab | CrossMabTM | Roche |
| scFv-Fab IgG | 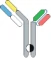 | Fab-Fc; ScFv-Fc | Vibecotamab; M802 | XmabTM (the engineered Fc to enhance the generation of heterodimeric Fc); YBODYTM | Xencor/Amgen; YZYBio |
| VH1-VH2-CH1-Fc1(G1) x VL2-VL1-CL-Fc2(G1) | 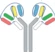 | 2 binding motif in one half antibody | SAR440234 | CODV-IgTM | Sanofi |
| VL1-CL1-VH2-CH2-Fc x VH1-CH1 x VL2-CL2 | 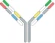 | 2 binding motif in one half antibody | EMB-01 | FIT-IgTM | EPIMAB BIOTHERAPEUTICS |
| VH-1-TCR Cα x VL-1-TCR Cβ; VH-2-CH-2-Fc x VL-2-CL-2 |  | KIH technology; TCR Cα/Cβ is used to substitute the CH1 and CL domain in one arm | WuXibodyTM | WuXi Biologics | |
| C-terminal linker of Fc |  | Link the other molecules at the C-terminal of Fc | APVO442 | ADAPTIR-FLEXTM | Aptevo Therapeutics |
| Fc antigen binding site |  | 2 natural binding sites; 2 additional binding sites in the Fc loop | FS118 | mAb2 | F-star Therapeutics |




