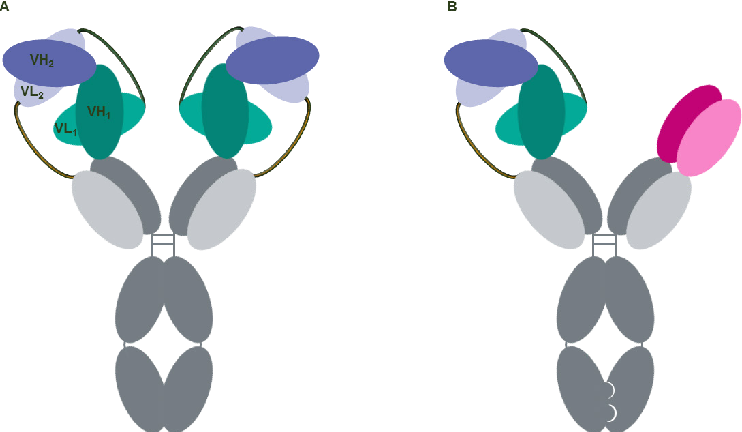
Format of bispecific antibodies (BsAbs)-VH1-VH2-CH1-Fc1(G1) x VL2-VL1-CL-Fc2(G1)
Virus-like particles (VLP) Platforms for immunogens, vaccines and drug carriers
Antibody-drug Conjugate (ADC): Pre-made ADC benchmark, MOA, Production and QC
Neutralizing antibodies of virus (SARS2, HIV, HBV, Rabies, RSV, Ebola, Influenza)
Immunoglobulin Fc receptors for Fc&Fc Receptor binding assay
ILIBRA-HuEasy Monoclonal antibody (mab) humanization service (fully humanized ab)
Single domain antibody (Nanobody)
SOCAIL MEDIA

CODV (cross-over dual variable domains) -Ig, contain four polypeptide chains that form two dual variable domains (four antigen binding sites) with a cross-over orientation (Figure 1), which is attained by inverting the alignment of the cognate domains on one chain. In order to adopt the correct VH/VL pairing, linker combinations were designed and optimized using a molecular modeling strategy. The overall CODV structure replicates a circular self-contained architecture (Figure 1A), with binding sites facing up to opposite sites and able to accommodate a large variety of antigen sizes while maintaining parental affinities. The CODV structure was also further developed in a trispecific format in which a single IgG Fab arm is combined with a double arm generated in the CODV structure using the KiH heterodimerization strategy (Figure 1B). A trispecific CODV molecule was successfully engineered to target three distinct epitopes on human immunodeficiency virus HIV-1 envelope, including the CD4 binding site, MPER and the V1V2 glycan site. This innovative molecule exhibited an unprecedented neutralization breadth and potency against HIV when compared to other previously described broadly neutralizing antibodies (BnAbs).
Formats of bispecific antibodies (BsAbs)
Many formats have been developed for BsAb generation as listed in the following table.
| Format | Schematic structure | Description | Example BsAb | Trademark | Company |
|---|---|---|---|---|---|
| tandem VHH | Tandem VHH fragment-based BsAb | N/A | |||
| tandem scFv |  | Tandem ScFv fragment-based BsAb | AMG330 | BiTETM | Amgen |
| Dual-affinity re-targeting antibody |  | Tandem domain-exchanged Fv (can also be used to fuse with Fc domain to create whole Abs) | Flotetuzumab | DARTTM | Macrogenics |
| Diabody |  | dimer of single-chain Fv (scFv) fragment | vixtimotamab | ReSTORETM | Amphivena Therapeutics |
| (scFv)2-Fab |  | a Fab domain and two scFv domains bind | A-337 | ITabTM | Generon/EVIVE Biotech |
| Rat–mouse hybrid IgG |  | Full-size IgG-like half antibodies from two different species | Catumaxomab | TriomabTM | Trion Pharma |
| Hetero heavy chain, Common light chain |  | Hetero heavy chain, Common light chain | Emicizumab | ART-IgTM | Genentech/ Chugai/Roche |
| Controlled Fab arm exchange | 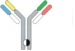 | Recombin the parental half antibodies | JNJ-64007957 | DuobodyTM | Genmab/ Janssen |
| Hetero H, forced HL IgG1 | 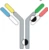 | KIH technology for heterodimerization of 2 distinct H chains, replacing the native disulfide bond in one of the CH1-CL interfaces with an engineered disulfide bond to enhance the cognate of H and L paring | MEDI5752 | DuetMabTM | MedImmune/ AstraZeneca |
| cH IgG1 | 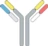 | Identical heavy chains; 2 different light chains: one kappa (κ) and one lambda (λ) | NI-1701 | κλ bodyTM | Novimmune SA |
| Hetero H, CrossMab | 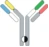 | KIH technology; domain crossover of immunoglobulin domains in the Fab region | Vanucizumab | CrossMabTM | Roche |
| scFv-Fab IgG | 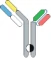 | Fab-Fc; ScFv-Fc | Vibecotamab; M802 | XmabTM (the engineered Fc to enhance the generation of heterodimeric Fc); YBODYTM | Xencor/Amgen; YZYBio |
| VH1-VH2-CH1-Fc1(G1) x VL2-VL1-CL-Fc2(G1) | 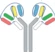 | 2 binding motif in one half antibody | SAR440234 | CODV-IgTM | Sanofi |
| VL1-CL1-VH2-CH2-Fc x VH1-CH1 x VL2-CL2 | 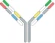 | 2 binding motif in one half antibody | EMB-01 | FIT-IgTM | EPIMAB BIOTHERAPEUTICS |
| VH-1-TCR Cα x VL-1-TCR Cβ; VH-2-CH-2-Fc x VL-2-CL-2 |  | KIH technology; TCR Cα/Cβ is used to substitute the CH1 and CL domain in one arm | WuXibodyTM | WuXi Biologics | |
| C-terminal linker of Fc |  | Link the other molecules at the C-terminal of Fc | APVO442 | ADAPTIR-FLEXTM | Aptevo Therapeutics |
| Fc antigen binding site |  | 2 natural binding sites; 2 additional binding sites in the Fc loop | FS118 | mAb2 | F-star Therapeutics |




